What does the Beer-Lambert Law relate?
Temperature and pressure
Concentration and absorbance
Volume and density
Mass and acceleration

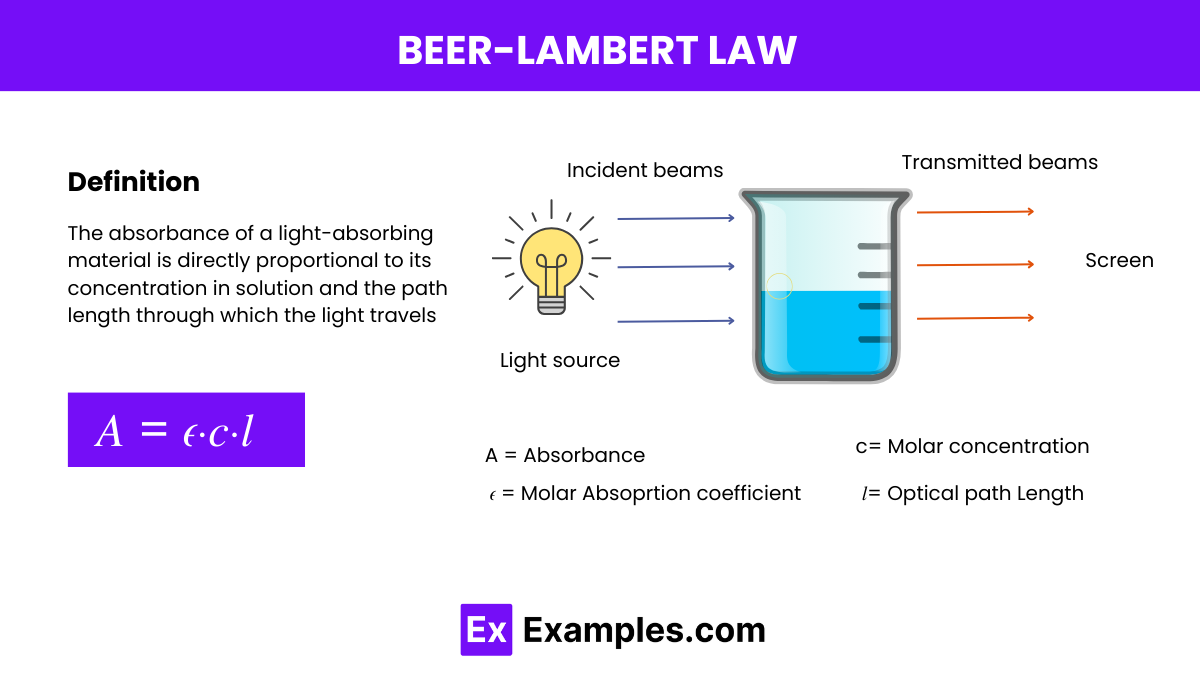
The Beer-Lambert Law, also known simply as Beer’s Law, is a fundamental statement in spectroscopy that relates the absorption of light to the properties of the material through which the light is traveling. The law states:
This law provides a quantitative basis for analyzing the concentration of an absorbing species in solution by measuring how much light is absorbed at a specific wavelength as it passes through a given path length of the solution.
Beer’s Law, also known as the Beer-Lambert Law, is a fundamental relationship that describes the absorption of light by a substance in solution. It quantitatively establishes how the absorption of light by a medium is directly proportional to the concentration of the absorbing substance and the path length of the light through that medium. The mathematical expression of Beer’s Law is:
Lambert’s Law, also known as Lambert-Beer Law or simply Beer’s Law when combined with Beer’s formulation, is a principle in optics and spectroscopy. It states that the absorbance of light by a substance in solution is directly proportional to the concentration of the substance and the path length of the light through the solution. The formula for Lambert’s Law is:
The formula for the Beer-Lambert Law, which describes the relationship between the absorbance of light by a solution and the properties of that solution, is given by:
This formula allows you to calculate the absorbance of light by a solution, providing a means to determine the concentration of an unknown sample based on its light absorption properties.
The Beer-Lambert Law is extensively used in chemistry, biology, and physics to determine the concentration of absorbing species in solution. Here are several practical examples of its application:
One of the most common applications of the Beer-Lambert Law is in analytical chemistry for determining the concentration of a chemical species in solution. By measuring the absorbance at a known wavelength, chemists can use the formula to calculate the concentration of the solution. This is particularly useful in titrations, where the concentration of an unknown solution is determined by comparing its absorbance to a standard curve of known concentrations.
In medical diagnostics, the Beer-Lambert Law is used to measure blood oxygen levels and other biochemical components. For instance, pulse oximetry, a non-invasive method that measures the oxygen saturation level of the blood, relies on the principles of this law by analyzing the absorption of light through capillary blood at different wavelengths.
Environmental scientists use the Beer-Lambert Law to monitor water quality by measuring the concentration of pollutants. Spectrophotometry, which is based on this law, helps in detecting and quantifying substances like heavy metals and organic compounds in water samples.
The law is also applied in the food and beverage industry to determine the concentration of colorants and additives in products. For example, the concentration of food dyes can be quantified by measuring the absorbance of light passing through a solution of the dye.
In pharmaceutical development and quality control, the Beer-Lambert Law is utilized to assess the purity and concentration of solutions. Measuring the absorbance of light by drug solutions helps in ensuring that each batch meets the required standards.
Biologists apply this law to measure concentrations of different biomolecules in research. For example, the quantification of protein concentrations using spectrophotometric assays like the Bradford protein assay often involves principles derived from the Beer-Lambert Law.
Quantifying the concentration of a solute in a solution by measuring the absorbance of light at a specific wavelength.
Assessing the concentration of drugs in pharmaceutical formulations for quality control purposes.
These examples demonstrate the versatility and critical importance of the Beer-Lambert Law across various scientific disciplines and industries.
1.Chemical Interactions: The law assumes that the absorbing species are independent of each other and do not interact. However, in real-world scenarios, interactions such as dimerization, association, or dissociation can occur, affecting the absorbance and leading to deviations from the expected linear relationship.
2.High Concentration Effects : At high concentrations, the solute molecules can be close enough to affect each other’s absorption properties. This can lead to deviations due to changes in refractive index, molecular aggregation, or reabsorption of light within the medium. The Beer-Lambert Law assumes that absorbance is linearly related to concentration, which may not hold true at higher concentrations.
3.Stray Light : Stray light in the spectrophotometer can cause errors in the measurement of absorbance, particularly at high absorbance values where the transmitted light is very low. This effect can lead to non-linear responses that violate the assumptions of the Beer-Lambert Law.
4.Polychromatic Light: The law is ideally applied under conditions where monochromatic light (light of a single wavelength) is used. If polychromatic light (light containing multiple wavelengths) is used, each component of the light might be absorbed differently, leading to inaccuracies unless corrections are made.
5.Scattering of Light : If the solution contains particles that can scatter light (such as a colloidal suspension), the scattered light can contribute to the apparent absorbance. This effect is particularly significant in biological samples, which often contain particles that scatter light.
6.Path Length Variations : The law assumes a uniform path length for all light rays passing through the sample. In practice, irregularities in the cuvette (container) or the positioning of the sample can lead to variations in path length, affecting the accuracy of the absorbance measurement.
7.Non-homogeneous Samples : Inhomogeneities within the sample, such as temperature gradients or concentration variations, can also affect the absorbance measurements, leading to errors if the sample does not have uniform properties throughout.
The Beer-Lambert law is used to measure and analyze the concentration of substances by assessing the light absorbance of solutions at specific wavelengths in spectroscopy.
The Beer-Lambert law is crucial for quantitative analysis in chemistry and biology, providing a direct method for determining the concentrations of absorbing substances in solutions.
Three key factors that influence the Beer-Lambert law are the concentration of the solution, the path length of the light through the solution, and the wavelength of the light used.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does the Beer-Lambert Law relate?
Temperature and pressure
Concentration and absorbance
Volume and density
Mass and acceleration
What is the formula for the Beer-Lambert Law?
A = ϵcl
A = c/ϵl
A = ϵ + cl
A = l/ϵc
In the Beer-Lambert Law, what does ϵ represent?
Path length
Concentration
Absorbance
Molar absorptivity
What is the unit of absorbance in the Beer-Lambert Law?
No unit (dimensionless)
Moles per liter (M)
Centimeters (cm)
Joules (J)
If the concentration of a solution is doubled, what happens to the absorbance according to the Beer-Lambert Law?
It halves
It remains the same
It doubles
It quadruples
Which component in the Beer-Lambert Law equation represents the distance light travels through the solution?
A
ϵ
c
l
What would be the effect on absorbance if the path length is increased?
Absorbance decreases
Absorbance remains the same
Absorbance increases
Absorbance fluctuates
A solution has an absorbance of 0.5, molar absorptivity of 1 L/(mol·cm), and path length of 1 cm. What is the concentration of the solution?
0.5 M
0.05 M
1 M
5 M
Which of the following is NOT a factor in the Beer-Lambert Law?
Path length
Concentration
Absorbance
Temperature
For a given concentration and path length, which property of the solute primarily affects the absorbance?
Molar absorptivity
Density
Volume
Pressure
Before you leave, take our quick quiz to enhance your learning!

