What does BTU stand for?
British Temperature Unit
British Thermal Unit
Basic Thermal Unit
Basic Temperature Unit

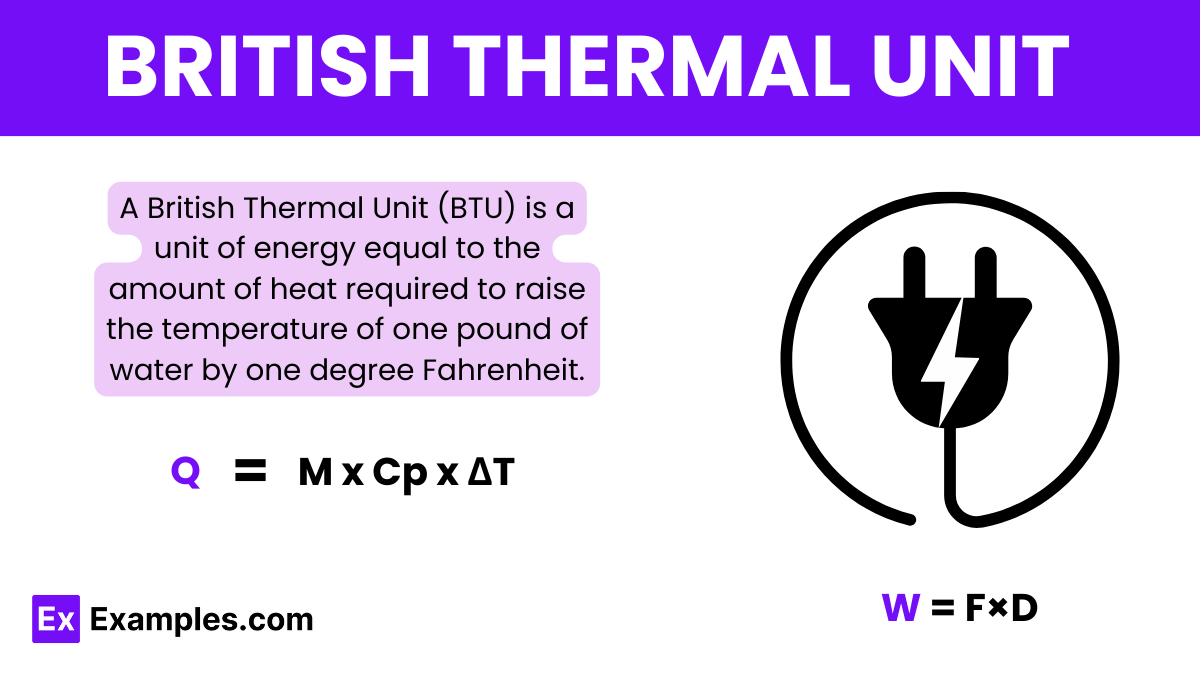
Q = M x Cp x ΔT
The formula to calculate British thermal units (BTUs) is:
BTU=mass×specific heat×Δtemperature
Where:
The basic formula for work and energy involving British thermal units (BTUs) is:
Where:
Kinetic Energy: The energy of an object in motion is known as kinetic energy, calculated by the formula: KE=1/2 mv² Where:
Potential Energy:The potential energy formula involving British thermal units (BTUs) is not typically defined in the same way as it is in classical mechanics, where potential energy is associated with the position of an object relative to a reference point.In this context, calculated by using the formula: PE = MxCxΔT Where:
| Prefix | Symbol | Multiplication Factor (BTU) |
|---|---|---|
| Yotta- | Y | 10²⁴ |
| Zetta- | Z | 10²¹ |
| Exa- | E | 10¹⁸ |
| Peta- | P | 10¹⁵ |
| Tera- | T | 10¹² |
| Giga- | G | 10⁹ |
| Mega- | M | 10⁶ |
| Kilo- | k | 10³ |
| Milli- | m | 10⁻³ |
| Micro- | μ | 10⁻⁶ |
| Nano- | n | 10⁻⁹ |

| From/To | Conversion Factor | Example |
|---|---|---|
| British Thermal Unit (BTU) to Joule (J) | 1 BTU = 1055.06 J | 1 BTU = 1055.06 Joules |
| British Thermal Unit (BTU) to Calorie (cal) | 1 BTU = 252.164 cal | 1 BTU = 252.164 calories |
| British Thermal Unit (BTU) to Kilowatt-hour (kWh) | 1 BTU = 0.000293 kWh | 1 BTU = 0.000293 kilowatt-hours |
| British Thermal Unit (BTU) to Electronvolt (eV) | 1 BTU = 6.585×10¹⁵ eV | 1 BTU = 6.585×10¹⁵ electronvolts |
| British Thermal Unit (BTU) to Watt-hour (Wh) | 1 BTU = 0.293071 Wh | 1 BTU = 0.293071 watt-hours |
| British Thermal Unit (BTU) to Megajoule (MJ) | 1 BTU = 1.05506 MJ | 1 BTU = 1.05506 megajoules |
| British Thermal Unit (BTU) to Gigajoule (GJ) | 1 BTU = 1.05506 × 10⁻⁶ GJ | 1 BTU ≈ 1.05506×10⁻⁶ gigajoules |
Convert BTUs to Joules, the standard unit of energy in the International System of Units (SI).
Convert BTUs to Calories, commonly used in nutrition and thermal energy calculations.
Convert BTUs to Kilowatt-hours, a measure of electrical energy consumption.
Convert BTUs to Electronvolts, a unit of energy commonly used in atomic and particle physics.
Convert BTUs to Watt-hours, another measure of electrical energy commonly used in households and industries.
Convert BTUs to Megajoules, a unit of energy used in engineering and thermodynamics.
Convert BTUs to Gigajoules, a larger unit of energy commonly used in the energy industry and power generation.
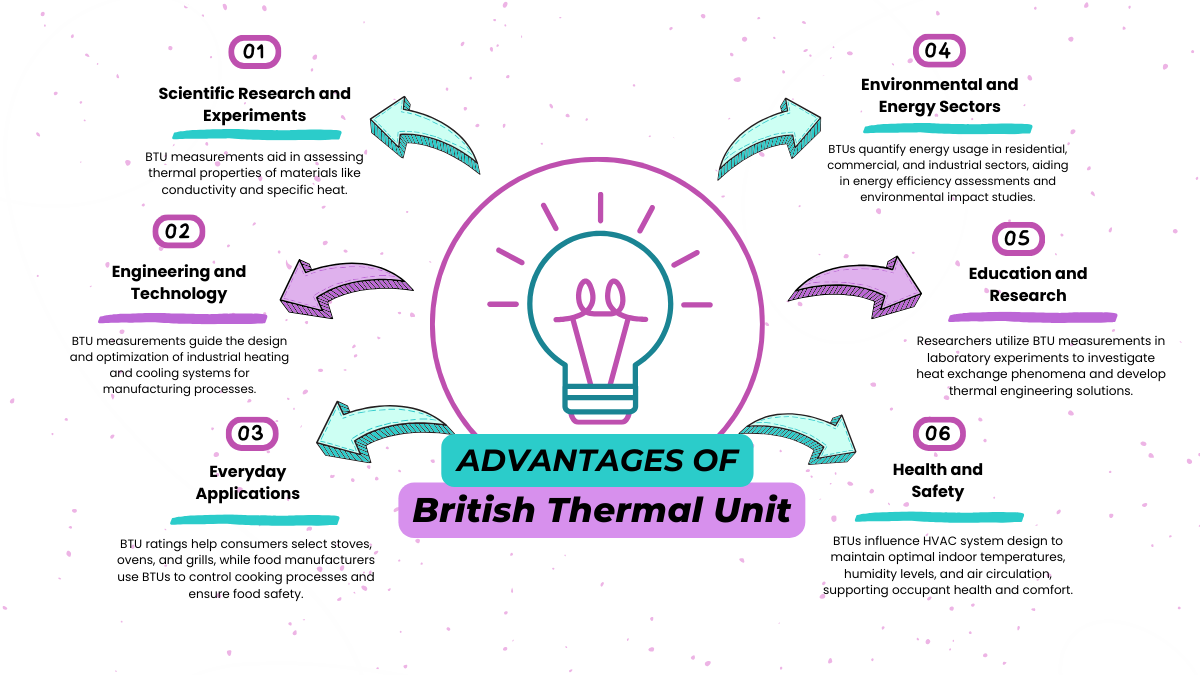
For instance, when selecting an air conditioner for a room, the BTU rating indicates how much heat the unit can remove from the air per hour. A higher BTU rating typically means the air conditioner has a greater cooling capacity and can effectively cool larger spaces.
In residential or commercial buildings, understanding the BTU requirements helps in choosing HVAC systems that are appropriately sized for the space, ensuring optimal comfort and energy efficiency.
Mechanical Work:
Electrical Energy:
Thermal Energy:
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does BTU stand for?
British Temperature Unit
British Thermal Unit
Basic Thermal Unit
Basic Temperature Unit
What is the definition of one BTU?
The amount of heat required to raise the temperature of 1 pound of water by 1°F
The amount of heat required to raise the temperature of 1 kilogram of water by 1°C
The amount of heat required to raise the temperature of 1 gram of water by 1°F
The amount of heat required to raise the temperature of 1 liter of water by 1°C
How many BTUs are approximately equivalent to 1 kilowatt-hour (kWh) of energy?
2.5 BTU
3412 BTU
5000 BTU
860 BTU
Which of the following appliances is typically rated in BTUs?
Light bulb
Air conditioner
Washing machine
Refrigerator
How many BTUs are required to raise the temperature of 10 pounds of water by 1°F?
1 BTU
5 BTU
10 BTU
100 BTU
What is the equivalent of 1 BTU in joules (J)?
4.2 J
42 J
105.5 J
1000 J
Which unit of energy is larger, 1 BTU or 1 calorie?
1 BTU
1 calorie
They are equal
It depends on the context
What is the primary use of BTU in the HVAC industry?
Measuring electrical consumption
Measuring heat energy
Measuring water flow
Measuring air pressure
If a room heater has a capacity of 5000 BTUs per hour, how much heat energy does it produce in 2 hours?
2500 BTU
5000 BTU
10000 BTU
20000 BTU
Which fuel type is commonly measured in BTUs per pound?
Electricity
Natural gas
Wood
Diesel
Before you leave, take our quick quiz to enhance your learning!

