Which of the following is considered a fundamental constant in physics?
Speed of light in a vacuum
Number of atoms in a mole
Gravitational acceleration on Earth
Density of water

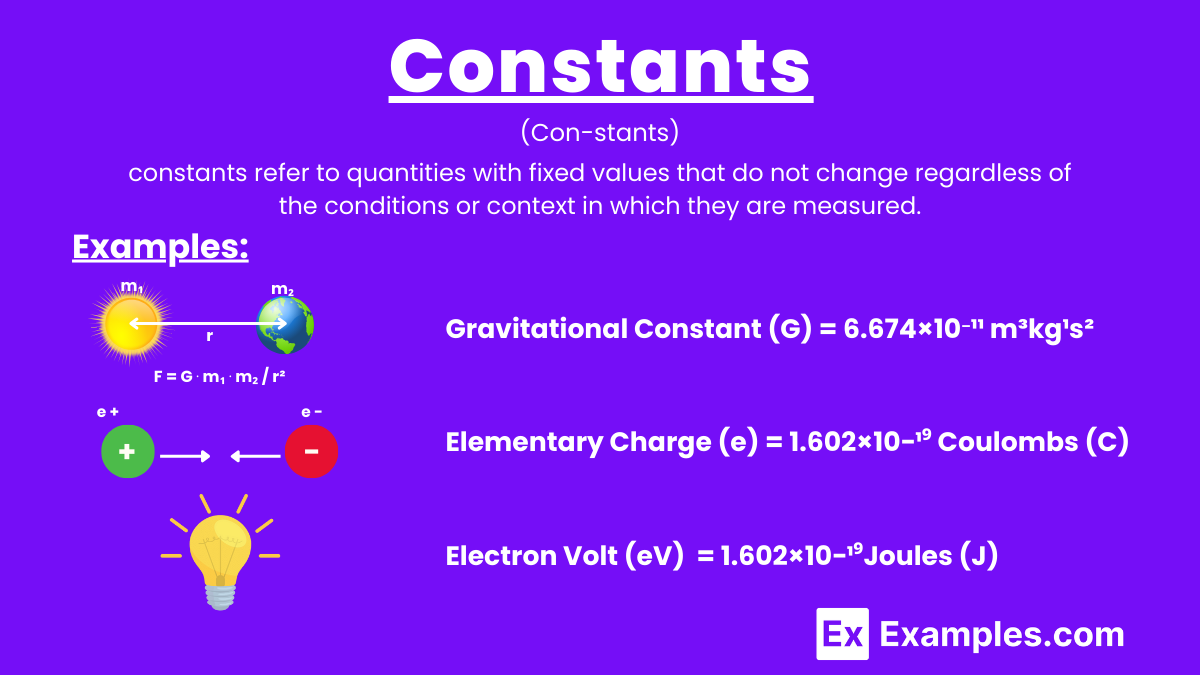
A constant in physics is a quantity with a fixed value that does not change regardless of the conditions or the variables in a given situation. Constants serve as fundamental benchmarks in various equations and theories, providing consistency and predictability in scientific calculations. Examples include the speed of light in a vacuum (c), Planck’s constant (h), and the gravitational constant (G). These constants are crucial for formulating physical laws and principles, enabling scientists to describe and understand the natural world accurately.
In physics, constants refer to quantities with fixed values that do not change regardless of the conditions or context in which they are measured. These values remain unchanged over time and space, serving as fundamental reference points in scientific calculations and theories.
| Constant | Symbol | Value | Units |
|---|---|---|---|
| Speed of Light | c | 2.998×10⁸ | meters per second (m/s) |
| Gravitational Constant | G | 6.674×10⁻¹¹ | m³kg¹s² |
| Planck’s Constant | h | 6.626×10−34 | Joule seconds (Js) |
| Reduced Planck’s Constant | ℏ | 1.055×10−34 | Joule seconds (Js) |
| Elementary Charge | e | 1.602×10−19 | Coulombs (C) |
| Avogadro’s Number | NA | 6.022×1023 | mol−1 |
| Boltzmann Constant | kB | 1.381×10−23 | Joules per kelvin (J/K) |
| Gas Constant | R | 8.314 | J ⋅ mol−1 ⋅ K−1 |
| Permittivity of Free Space | ε0 | 8.854×10−12 | F⋅m−1 |
| Permeability of Free Space | μ0 | 4π×10−7 | N⋅A−2 |
| Electron Mass | me | 9.109×10−31 | Kilograms (kg) |
| Proton Mass | mp | 1.673×10−27 | Kilograms (kg) |
| Neutron Mass | mn | 1.675×10−27 | Kilograms (kg) |
| Fine-Structure Constant | α | 7.297×10−3 | Dimensionless |
| Stefan-Boltzmann Constant | σ | 5.670×10−8 | W⋅m−2⋅K−4 |
| Rydberg Constant | R∞ | 1.097×107 | m−1 |
| Faraday Constant | F | 9.649×104 | Coulombs per mole (C/mol) |
| Atomic Mass Unit | u | 1.661×10−27 | Kilograms (kg) |
| Bohr Radius | a0 | 5.292×10−11 | Meters (m) |
| Coulomb’s Constant | ke | 8.988×109 | N⋅m2⋅C−2 |
| Universal Gas Constant | R | 8.314 | J⋅mol−1⋅K−1 |
| Planck Length | ℓP | 1.616×10−35 | Meters (m) |
| Planck Time | tP | 5.391×10−44 | Seconds (s) |
| Planck Mass | mP | 2.176×10−8 | Kilograms (kg) |
| Planck Temperature | TP | 1.416×1032 | Kelvin (K) |
| Wien’s Displacement Constant | b | 2.898×10−3 | m⋅K |
| Bohr Magneton | μB | 9.274×10−24 | Joules per Tesla (J/T) |
| Nuclear Magneton | μN | 5.051×10−27 | Joules per Tesla (J/T) |
| Magnetic Flux Quantum | Φ0 | 2.068×10−15 | Weber (Wb) |
| Conductance Quantum | G0 | 7.748×10−5 | Siemens (S) |
| Von Klitzing Constant | RK | 2.581×104 | Ohms (Ω) |
| Josephson Constant | KJ | 4.835×1014 | Hz⋅V−1 |
| Compton Wavelength | λC | 2.426×10−12 | Meters (m) |
| Electron Volt | eV | 1.602×10−19 | Joules (J) |
| Hartree Energy | Eh | 4.360×10−18 | Joules (J) |
| Atomic Unit of Charge | e | 1.602×10−19 | Coulombs (C) |
| Atomic Unit of Mass | me | 9.109×10−31 | Kilograms (kg) |
| Atomic Unit of Length | a0 | 5.292×10−11 | Meters (m) |
| Atomic Unit of Time | t0 | 2.418×10−17 | Seconds (s) |
| Fermi Coupling Constant | GF | 1.166×10−5 | GeV−2 |
| Weak Mixing Angle | sin2θW | 0.2229 | Dimensionless |
| Solar Mass | M⊙ | 1.989×1030 | Kilograms (kg) |
| Astronomical Unit | AU | 1.496×1011 | Meters (m) |
| Light Year | ly | 9.461×1015 | Meters (m) |
| Parsec | pc | 3.086×1016 | Meters (m) |
| Hubble Constant | H0 | 67.4 | km⋅s−1⋅Mpc−1 |
| Chandrasekhar Limit | MCh | 1.4 | Solar Masses (M_\odot) |
| Electron Magnetic Moment | μe | −9.284×10−24 | Joules per Tesla (J/T) |
The speed of light in a vacuum, denoted as c, is approximately 2.998×10⁸ meters per second. This constant is fundamental in physics because it sets the maximum speed at which all energy, matter, and information in the universe can travel. It plays a crucial role in Einstein’s theory of relativity, which shows how space and time are interwoven.
Example: Light from the Sun takes about 8 minutes and 20 seconds to reach Earth, traveling at this constant speed.
The gravitational constant, G, is 6.674×10⁻¹¹ m³kg¹s². It is a key quantity in Newton’s law of universal gravitation, which describes the gravitational attraction between two masses. This constant helps us understand the strength of the gravitational force in the universe.
Example: The gravitational force between two 1 kg masses separated by 1 meter is 6.674×10−11 Newtons.
Planck’s constant, h, is 6.626×10−34 Joule seconds. It is a fundamental constant in quantum mechanics, reflecting the quantization of energy levels in atomic and subatomic systems. It is central to the Heisenberg uncertainty principle and the Planck-Einstein relation E=hν, linking energy and frequency.
Example: The energy of a photon with a frequency of 5×1014 Hz is 3.313×10−19 Joules, calculated using E=hν.
The reduced Planck’s constant, ℏ, is 1.055×10−34 Joule seconds. It is used frequently in quantum mechanics and is equal to Planck’s constant divided by 2π. It is pivotal in defining the scales at which quantum effects become significant.
Example: The angular momentum of an electron in the ground state of a hydrogen atom is ℏ.
The elementary charge, e, is 1.602×10−19 Coulombs. This constant represents the electric charge carried by a single proton or the magnitude of the charge of a single electron, crucial for understanding electromagnetic interactions.
Example: The charge of an electron is −1.602×10−19 Coulombs.
Avogadro’s number, NA, is 6.022×10 23 per mole. It defines the number of atoms, ions, or molecules in one mole of a substance, forming a bridge between the macroscopic and atomic worlds in chemistry and physics.
Example: One mole of water molecules contains 6.022×1023 water molecules.
The Boltzmann constant, kB, is 1.381×10−23 Joules per Kelvin. It relates the average kinetic energy of particles in a gas with the temperature of the gas, playing a crucial role in statistical mechanics and thermodynamics.
Example: At room temperature (300 K), the average kinetic energy of a gas molecule is 4.143×10−21
Joules.
The gas constant, R, is 8.314 J⋅mol−1⋅K−1. It is the constant of proportionality in the ideal gas law PV=nRT, linking pressure, volume, temperature, and the amount of gas.
Example: The pressure of 1 mole of an ideal gas at 1 liter and 300 K is 24.942 atm.
The permittivity of free space, ε0, is 8.854×10−12 F⋅m−1. It is a measure of the ability of the vacuum to permit electric field lines, essential for understanding electrostatics and capacitance.
Example: The capacitance of a parallel-plate capacitor with a vacuum between the plates, an area of 1m2, and a separation of 1m is 8.854×10−9 Farads.
The permeability of free space, μ0, is 4π×10−7. This constant measures the ability of the vacuum to support magnetic field lines, important in the study of magnetostatics and electromagnetic theory.
Example: The magnetic field around a long straight wire carrying a current of 1 A is 2×10−7 Tesla at a distance of 1 meter.
The electron mass, me, is 9.109×10−31 kilograms. This is the mass of a single electron at rest and is a fundamental constant in both atomic physics and quantum mechanics.
Example: The rest energy of an electron is 8.187×10−14 Joules, calculated using E=mc2
The proton mass, mp, is 1.673×10−27 kilograms. It represents the mass of a single proton, fundamental in nuclear physics and chemistry, as protons are a primary constituent of atomic nuclei.
Example: The rest energy of a proton is 1.503×10−10 Joules.
The neutron mass, mn, is 1.675×10−27 kilograms. Neutrons, along with protons, make up the atomic nucleus, and their mass is crucial for calculations involving nuclear reactions and stability.
The fine-structure constant, α, is 7.297×10−3. This dimensionless constant characterizes the strength of the electromagnetic interaction between elementary charged particles, fundamental in quantum electrodynamics.
The Stefan-Boltzmann constant, σ is 5.670×10−8 W⋅m−2⋅K−4. It relates the total energy radiated per unit surface area of a black body to the fourth power of its temperature, crucial in thermodynamics and astrophysics.
The Rydberg constant, R∞, is 1.097×107 m−1. It is used in atomic physics to describe the wavelengths of spectral lines of many chemical elements.
The Faraday constant, F, is 9.649×104 Coulombs per mole. It represents the charge of one mole of electrons, linking electrochemistry to the amount of substance.
The atomic mass unit, u, is 1.661×10−27 kilograms. It is used to express atomic and molecular masses, providing a convenient scale for comparison of different atoms and molecules.
The Bohr radius, a0, is 5.292×10−11 meters. It represents the average distance between the nucleus and the electron in a hydrogen atom in its ground state, fundamental in atomic physics.
Coulomb’s constant, ke, is 8.988×109 N⋅m2⋅C−2. It is the proportionality constant in Coulomb’s law, describing the force between two point charges.
The universal gas constant, R, is 8.3148.3148.314 J⋅mol−1⋅K−1\text{J} \cdot \text{mol}^{-1} \cdot \text{K}^{-1}J⋅mol−1⋅K−1. It appears in the ideal gas law and other equations of state, relating the macroscopic properties of gases.
The Planck length, ℓP, is 1.616×10−35 meters. It is a fundamental scale in quantum mechanics, representing the smallest meaningful length, where classical ideas about gravity and space-time cease to be valid.
The Planck time, tP, is 1.416×1032 seconds. It is the time it takes for light to travel one Planck length, significant in quantum gravity and cosmology.
The Planck mass, mP, is 2.176×10−8 kilograms. It is a fundamental mass scale in quantum mechanics, marking the transition between classical and quantum descriptions of gravity.
The Planck temperature, TP, is 1.416×1032 Kelvin. It is the highest theoretically possible temperature, beyond which the laws of physics as currently understood cease to be useful.
Wien’s displacement constant, b, is 2.898×10−3 m.k. It relates the temperature of a black body to the wavelength at which it emits radiation most strongly, used in thermal radiation studies.
The Bohr magneton, μB, is 9.274×10−24 Joules per Tesla. It is a physical constant related to the magnetic moment of an electron due to its orbital or spin motion, important in quantum mechanics and magnetism.
The nuclear magneton, μN, is 5.051×10−27 Joules per Tesla. It is similar to the Bohr magneton but smaller, used to describe the magnetic moment of nucleons and nuclei.
The magnetic flux quantum, Φ0, is 2.068×10−15 Weber. It is the quantum of magnetic flux, fundamental in the study of superconductivity and quantum Hall effects.
The conductance quantum, G0, is 7.748×10−5 Siemens. It represents the quantized unit of electrical conductance, crucial in the study of quantum transport.
The von Klitzing constant, RK, is 2.581×104 Ohms. It is used in the quantum Hall effect to define the resistance quantum, providing a standard for electrical resistance.
The Josephson constant, KJ, is 4.835×1014 Hz⋅V−1. It relates the voltage across a Josephson junction to the frequency of the resulting AC current, important in superconductivity.
The Compton wavelength, λC, is 2.426×10−12 meters. It represents a quantum mechanical limit to the localization of particles, significant in quantum field theory.
An electron volt, eV, is 1.602×10−19 Joules. It is a unit of energy commonly used in atomic, nuclear, and particle physics, representing the energy gained by an electron when accelerated through a potential difference of one volt.
The Hartree energy, Eh, is 4.360×10−18 Joules. It is a unit of energy used in atomic physics and quantum chemistry, representing the electrostatic potential energy of the hydrogen atom in its ground state.
The atomic unit of charge, e, is 1.602×10−19 Coulombs. It is used as a convenient unit of electric charge in atomic physics and quantum chemistry.
The atomic unit of mass, me, is 9.109×10−31 kilograms. It provides a convenient scale for comparing the masses of different particles in atomic and molecular systems.
The atomic unit of length, a0, is 5.292×10−11 meters. It is the Bohr radius, representing the typical size of atoms and providing a natural length scale in atomic physics.
The atomic unit of time, t0, is 2.418×10−17 seconds. It provides a natural time scale in atomic and molecular physics, corresponding to the period of an electron orbiting a hydrogen nucleus.
The Fermi coupling constant, GF, is 1.166×10−5 GeV−2. It characterizes the strength of the weak force, one of the four fundamental forces in the universe, crucial in particle physics.
The weak mixing angle, sin2θW, is 0.2229. It quantifies the mixing of the electromagnetic and weak forces in the electroweak interaction, fundamental in the Standard Model of particle physics.
The solar mass, M⊙, is 1.989×1030 kilograms. It is the mass of the Sun, used as a standard unit for expressing the masses of other stars and galaxies in astrophysics.
The astronomical unit, AU, is 1.496×1011 meters. It represents the average distance between the Earth and the Sun, providing a useful scale for measuring distances within the solar system.
A light year, ly, is 9.461×1015 meters. It represents the distance that light travels in a vacuum in one year, used to express astronomical distances.
A parsec, pc, is 3.086×1016 meters. It is a unit of distance used in astronomy, equal to about 3.26 light years, representing the distance at which one astronomical unit subtends an angle of one arcsecond.
The Hubble constant, H0, is 67.4 km⋅s−1⋅Mpc−1. It measures the rate of expansion of the universe, fundamental in cosmology.
The Chandrasekhar limit, MCh, is 1.4 solar masses. It is the maximum mass of a stable white dwarf star, beyond which it will collapse into a neutron star or black hole, significant in stellar evolution.
The electron magnetic moment, μe, is −9.284×10−24 Joules per Tesla. It represents the intrinsic magnetic moment of an electron due to its spin, fundamental in quantum mechanics and magnetism.
Constants provide a foundation for developing theories and equations, ensuring consistency and accuracy in scientific measurements and predictions.
The Coulomb constant (ke) is 8.987551787×109 N·m²/C², used in electrostatics to describe the force between two point charges.
he proton mass (𝑚𝑝) is 1.67262192369×10−27 kilograms, a fundamental constant in particle physics.
The mass of a neutron is approximately 1.674927498×10−27atomic mass units (amu).
The Hartree energy (Eh) is 4.359×10-18 joules, a unit of energy used in atomic physics and quantum chemistry.
The Compton wavelength (𝜆𝐶) is2.426×10−12 meters, representing the wavelength increase of a photon when scattered by a particle.
The Faraday constant (𝐹) is 96485.33212 C/mol, the total electric charge carried by one mole of electrons.
The Wien displacement constant (b) is 2.897771955×10−3 m·K, describing the relationship between the temperature of a blackbody and the wavelength at which it emits most strongly.
The universal gas constant (𝑅) is 8.3144621 J/mol·K, the constant in the equation of state of an ideal gas, relating energy scale to temperature scale.
The Rydberg constant (𝑅∞) is1.097373×107m−1, used in atomic physics to describe the wavelengths of spectral lines.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which of the following is considered a fundamental constant in physics?
Speed of light in a vacuum
Number of atoms in a mole
Gravitational acceleration on Earth
Density of water
What does the constant ccc represent in Einstein's equation E = mc²?
Speed of light
Speed of sound
Speed of gravity
Speed of electricity
What is the value of the gravitational constant \( G \)?
\( 6.674 \times 10^{-11} \, \text{m}^3 \text{kg}^{-1} \text{s}^{-2} \)
\( 9.81 \, \text{m/s}^2 \)
\( 8.314 \, \text{J/mol·K} \)
\( 1.602 \times 10^{-19} \, \text{C} \)
Which constant represents the charge of an electron?
Planck constant
Electron charge
Boltzmann constant
Avogadro's number
What is the Planck constant denoted as?
\( h \)
\( k \)
\( e \)
\( G \)
What is the constant used to measure the electric constant or permittivity of free space?
\( h \)
\( \mu_0 \)
\( \epsilon_0 \)
\( k_B \)
Which constant is used in Coulomb's law to quantify the electric force between two charges?
Gravitational constant
Coulomb constant
Planck constant
Gas constant
What does the constant \( \mu_0 \) represent?
Magnetic permeability of free space
Electric permittivity of free space
Speed of sound in air
Universal gas constant
The constant \( R \) represents which physical quantity?
Electric resistance
Atomic radius
Radius of curvature
Gas constant
Which constant is fundamental in the theory of relativity?
Speed of light
Universal gas constant
Boltzmann constant
Avogadro's number
Before you leave, take our quick quiz to enhance your learning!

