What does Coulomb's Law describe?
The force between two magnetic poles
The force between two electric charges
The force between two masses
The force within an atom
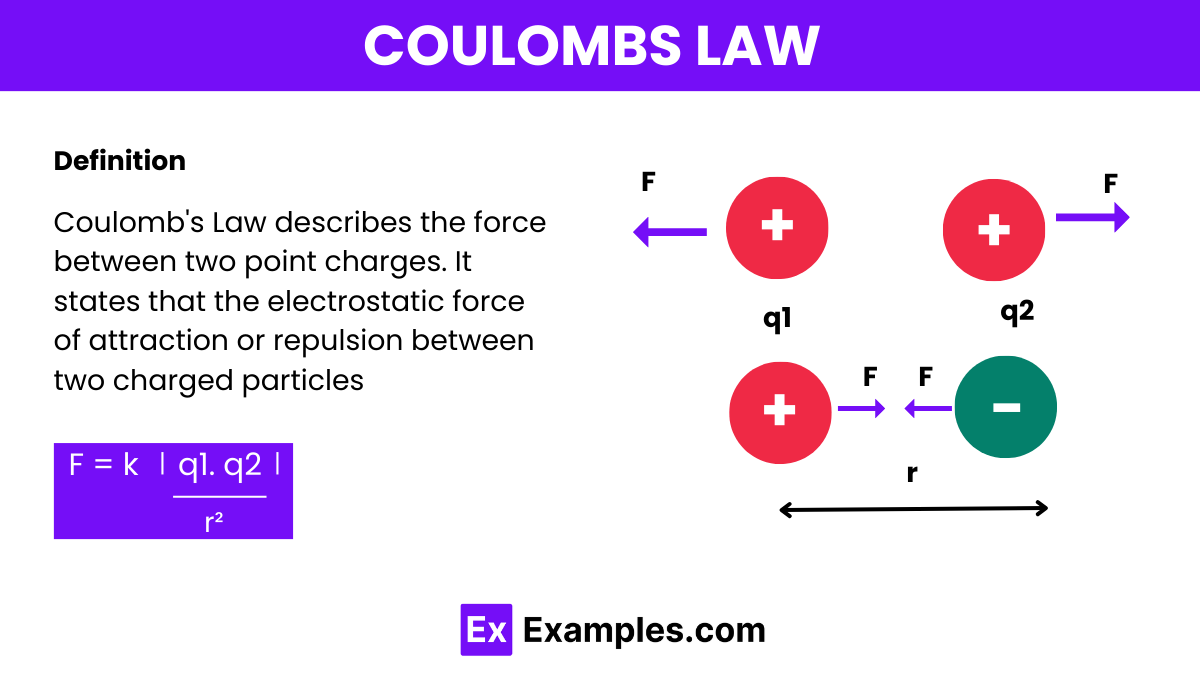
The formula for Coulomb’s Law quantifies the electrostatic force between two charged particles. It is expressed as:
Where:
F is the magnitude of the electrostatic force between the charges.
𝑞1 and 𝑞2 are the magnitudes of the electrical charges. 𝑟is the distance between the centers of the two charges.
𝑘 is Coulomb’s constant, approximately equal to 8.9875×10⁹ Nm²/ C² in a vacuum.
One real-life example of Coulomb’s Law is the operation of a photocopier or laser printer. These devices utilize the principles of electrostatic charges to transfer toner (fine powder) onto paper. Here’s how it works:
Charging: The drum or photoconductor unit in a photocopier is initially given a uniform electrostatic charge via a high voltage wire called a corona wire.
Exposure: An image of the document is projected onto the drum. The light from the image discharges certain areas of the drum, creating a pattern of electrical charges that corresponds to the original document.
Development: Toner, which is negatively charged, is attracted to the positively charged areas of the drum (where the light hit), due to electrostatic forces described by Coulomb’s Law.
Transfer and Fusing: The toner is then transferred to the paper through further electrostatic charges, and finally, the toner is fused to the paper with heat and pressure, creating a permanent image.
Attraction and Repulsion: These accumulated charges can exert forces on nearby charged or polarized objects. For example, a charged balloon can attract neutral objects like dust or small paper bits. The electrostatic force described by Coulomb’s Law explains this attraction: the electric field created by the charged balloon induces opposite charges on the near side of a neutral object and like charges on the far side, leading to an overall attraction (electrostatic induction).
Practical Use: This principle is utilized in electrostatic air cleaners, which charge dust particles as they pass through the cleaner. The charged particles are then attracted to plates with an opposite charge, effectively removing them from the air.
In this process, Coulomb’s Law explains how charged particles (toner) are attracted or repelled by other charges (charged areas on the drum), which is critical for the precise transfer of toner to create high-quality images.
Electronics
Coulomb’s Law is fundamental in designing electronic components like capacitors and electrostatic sensors. It helps in calculating the forces between charges, which is crucial for understanding how these devices store and sense electrical energy.
Environmental Engineering
In air pollution control, electrostatic precipitators use Coulomb’s Law to remove particles from exhaust streams. Charged plates create an electric field that attracts dust and other particulates, effectively cleaning the air.
Material Science
Coulomb’s Law is used in the manipulation and assembly of nanoscale materials. Electrostatic forces can be used to position nanoparticles precisely, enabling the construction of advanced materials and devices at the atomic level.
Printing Technology
In digital printing technologies like laser printers and photocopiers, Coulomb’s Law is used to manage the attraction of toner particles to charged drum units. This ensures precise image and text transfers onto paper.
Medical Devices
Electrostatic forces are used in some types of medical drug delivery systems, where charges help to control the release and targeting of medication within the body. This application relies on understanding the forces described by Coulomb’s Law.
Physics Research
Coulomb’s Law is crucial in experiments involving charged particles in fields like plasma physics and during the development of equipment such as particle accelerators, where understanding the interactions between charged particles is essential.
Semiconductor Technology
Coulomb’s Law helps in the design and functioning of semiconductor devices. Understanding how electrostatic forces affect electron flow is key to optimizing the performance of transistors and diodes.
Touchscreen Technology
In capacitive touchscreens found in smartphones and tablets, Coulomb’s Law is applied to detect changes in the electrostatic field caused by the user’s touch. This interaction helps the device register where the screen has been touched.
Coulomb’s Law is a critical principle within electromagnetism, but it does have certain limitations that restrict its applicability under specific conditions:
Point Charges: Coulomb’s Law is ideally accurate for point charges, where the charges are considered to be concentrated at a single point. In real-world scenarios involving extended bodies with distributed charges, the law must be applied with approximations or adjustments.
Static Conditions: The law applies strictly to static (non-moving) charges. When charges are in motion, magnetic fields are produced in addition to electric fields, and the interactions become significantly more complex, requiring the use of Lorentz force law and electromagnetic field theory.
Medium Dependency: Coulomb’s constant 𝑘 in the formula depends on the permittivity of the medium in which the charges exist. Coulomb’s Law as originally formulated applies directly in a vacuum. In other media, the electric force is influenced by the medium’s permittivity, which can diminish the force between charges.
Relativistic Effects: At very high velocities, close to the speed of light, relativistic effects must be considered. Coulomb’s Law does not account for these because it assumes that changes in the electric field propagate instantaneously to all distances. However, changes in electromagnetic fields propagate at the finite speed of light.
Quantum Mechanical Effects: In the quantum realm, where particle sizes are on the scale of atoms and subatomic particles, classical physics predictions, including those made by Coulomb’s Law, often fail to accurately describe interactions. Quantum electrodynamics (QED) provides a more precise framework in these cases.
These limitations mean that while Coulomb’s Law is extremely useful for understanding many aspects of electric forces and fields in everyday situations, it must be supplemented by additional theories and principles to fully describe the behavior of charges under all conditions.
Gauss’s law is more advanced than Coulomb’s law because it provides a unified approach to understanding electric fields by considering the distribution of charges, rather than just individual point charges.
Coulomb’s constant 𝑘is derived from the equation k= 1/ 4πϵ₀ where ϵ₀ is the permittivity of free space, a fundamental physical constant.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does Coulomb's Law describe?
The force between two magnetic poles
The force between two electric charges
The force between two masses
The force within an atom
According to Coulomb's Law, the force between two charges is directly proportional to which of the following?
The square of the distance between them
The product of the charges
The sum of the charges
The difference of the charges
If the distance between two charges is doubled, how does the force between them change?
It doubles
It halves
It becomes four times greater
It becomes one-fourth as great
Two charges of +3 C and −3 C are placed 1 meter apart. What is the nature of the force between them?
Attractive
Repulsive
Neutral
Zero
What happens to the force between two charges if one of the charges is tripled?
It remains the same
It is halved
It is tripled
It is squared
Two charges are separated by a distance r. If the force between them is F, what will be the force if the distance is reduced to half?
2F
4F
F/2
F/4
If the charge on each of two objects is doubled, how does the force between them change?
It remains the same
It is doubled
It is quadrupled
It is halved
Which of the following factors affects the magnitude of the electrostatic force between two charges?
The mass of the charges
The speed of the charges
The distance between the charges
The shape of the charges
What is the direction of the force between two like charges?
Attractive
Repulsive
Neutral
Perpendicular
Two charges are placed at a certain distance apart and experience a force F. If both charges are increased by a factor of 3, what will be the new force between them?
F
3F
6F
9F
Before you leave, take our quick quiz to enhance your learning!

