The Van der Waals equation is an adjustment of which ideal gas law equation?
PV = nRT
PV = \frac{nRT}{V - b}
P = \frac{nRT}{V - b} - \frac{a n^2}{V^2}
P = \frac{nRT}{V} - \frac{a}{V^2}


The Van der Waals equation is a significant enhancement of the ideal gas law, developed to account for the real behavior of gases. While the ideal gas law, (PV = nRT), assumes that gas molecules do not interact and occupy no volume, these assumptions fall short under high-pressure and low-temperature conditions. Johannes Diderik van der Waals introduced corrections to address these limitations. The derivation of the Van der Waals equation incorporates two essential factors: the finite size of gas molecules and the intermolecular attractive forces.
The equation introduces two parameters, (a) and (b), where (a) corrects for the intermolecular forces, and (b) accounts for the finite volume occupied by gas molecules. The corrected volume is represented as (V – nb), and the adjusted pressure includes an additional term, (an²/Van²), reflecting the intermolecular attractions. Combining these corrections with the ideal gas law, we obtain the Van der Waals equation:
[ (P + an²/V²) (V-nb) = nRT ]
This equation provides a more accurate description of gas behavior, particularly under non-ideal conditions, making it crucial for various scientific and industrial applications, from chemical engineering to environmental science.
The Van der Waals equation improves upon the ideal gas law by accounting for the real behavior of gases, particularly at high pressures and low temperatures. This derivation introduces two critical corrections to the ideal gas law: one for the volume occupied by gas molecules and another for the intermolecular forces between them.
1.Ideal Gas Law
PV = nRT
The Ideal Gas Law is given by:
where:
2. Correct for the Volume of Gas Molecules:
Real gas molecules occupy a finite volume, which becomes significant at high pressures. Let (b) be the volume excluded by a mole of gas due to the finite size of the molecules. Thus, the effective volume available for gas molecules to move in is (V – nb), where (n) is the number of moles:
Vₑբբₑ꜀ₜᵢᵥₑ = V – nb
3. Correct for Intermolecular Forces:
In real gases, attractive forces between molecules reduce the impact of collisions on the container walls, effectively reducing the pressure. The reduction in pressure is proportional to the square of the molar concentration of the gas, (n/b)², and the constant of proportionality is (a). Therefore, the corrected pressure is:
[Pₑբբₑ꜀ₜᵢᵥₑ = P + an²/V² ]
4. Combine the Corrections with the Ideal Gas Law:
Substitute the corrected pressure and volume into the ideal gas law:
(P + an²/V²) (V – nb) = nRT
5. Simplify the Expression:
Expand and rearrange the equation to make it clearer:
P(V – nb) + an²/V² (V-nb) = nRT
Simplifying further, we get:
P(V – nb) +an²/V – an³b/V² = NRT
6. Van der Waals Equation for One Mole of Gas:
For simplicity, consider one mole of gas ((n = 1)):
( P + a/V²) (V – b) = RT
7.General Form of Van der Waals Equation:
For (n) moles of gas, the equation becomes:
( P + an²/V²) (V – nb) = nRT
The Van der Waals equation is a thermodynamic equation of state that describes the behavior of real gases. It is an improvement upon the ideal gas law, which assumes that gas molecules do not interact and have no volume. The Van der Waals equation introduces corrections for these assumptions, providing a more accurate representation of gas behavior under various conditions, especially at high pressures and low temperatures.
The Van der Waals equation is expressed as:
( P + an²/V²) (V – nb) = nRT
where:
Pressure Correction (an²/V²):
Volume Correction (V – nb):
The Van der Waals equation is significant because it provides a more realistic model for the behavior of gases compared to the ideal gas law. It is especially useful in scenarios where gases are not ideal, such as at high pressures or low temperatures. Some applications include:
Here’s a step-by-step derivation of the Van der Waals equation for real gases:
The ideal gas law is given by:
[ PV = nRT ]
In reality, gas molecules occupy a finite volume. Therefore, the volume available for the gas molecules to move in is less than the volume of the container. Let ( b ) be the volume occupied by one mole of gas molecules. The corrected volume ( V’ ) is:
[ V’ = V – nb ]
where ( n ) is the number of moles.
Real gas molecules experience intermolecular attractions, which reduce the pressure exerted on the walls of the container. Van der Waals introduced a term to account for these attractions. Let ( a ) be a constant that measures the strength of these attractions. The corrected pressure ( P’ ) is:
( P’ = P + an²/V² )
Replace the pressure ( P ) and volume ( V ) in the ideal gas law with the corrected pressure ( P’ ) and corrected volume ( V’ ):
( P + an²/V² ) ( V – nb )
Simplifying the above expression, we obtain the Van der Waals equation for real gases:
( P + an²/V² ) ( V – nb )
To derive the Van der Waals equation for one mole of gas, we start with the Ideal Gas Law and introduce corrections for the finite size of gas molecules and intermolecular forces.
The ideal gas law relates pressure, volume, temperature, and moles of gas, assuming no intermolecular forces or molecular volume: ( PV = nRT ).
In a real gas, attractive intermolecular forces reduce the effective pressure exerted by the gas molecules on the walls of the container. This reduction in pressure can be accounted for by adding a term (\frac{a}{V_m^2}) to the pressure, where ( a ) is a constant specific to the gas that represents the magnitude of the intermolecular forces. Therefore, the corrected pressure is:
[PᵢDₑₐₗ = P +a/V²ₘ ]
In an ideal gas, the volume occupied by the gas molecules themselves is negligible. However, in a real gas, the finite size of the molecules means that not all of the volume of the container is available for the gas molecules to move around in. This is accounted for by subtracting a term ( b ), the volume occupied by one mole of gas molecules, from the molar volume ( V_m ). Therefore, the corrected volume is:
[ Vₑբբ = Vₘ – b ]
We now combine these corrections into the Ideal Gas Law. The corrected pressure ( PᵢDₑₐₗ ) and the corrected volume ( Vₑբբ ) are substituted into the Ideal Gas Law:
[ PᵢDₑₐₗ Vₑբբ = RT ]
Substitute ( PᵢDₑₐₗ and Vₑբբ ):
(P + a/V²ₘ) (Vₘ – b) = RT
This is the Van der Waals equation for one mole of a real gas.
Download Uses of the Derivation of the Van der Waals Equation Pdf
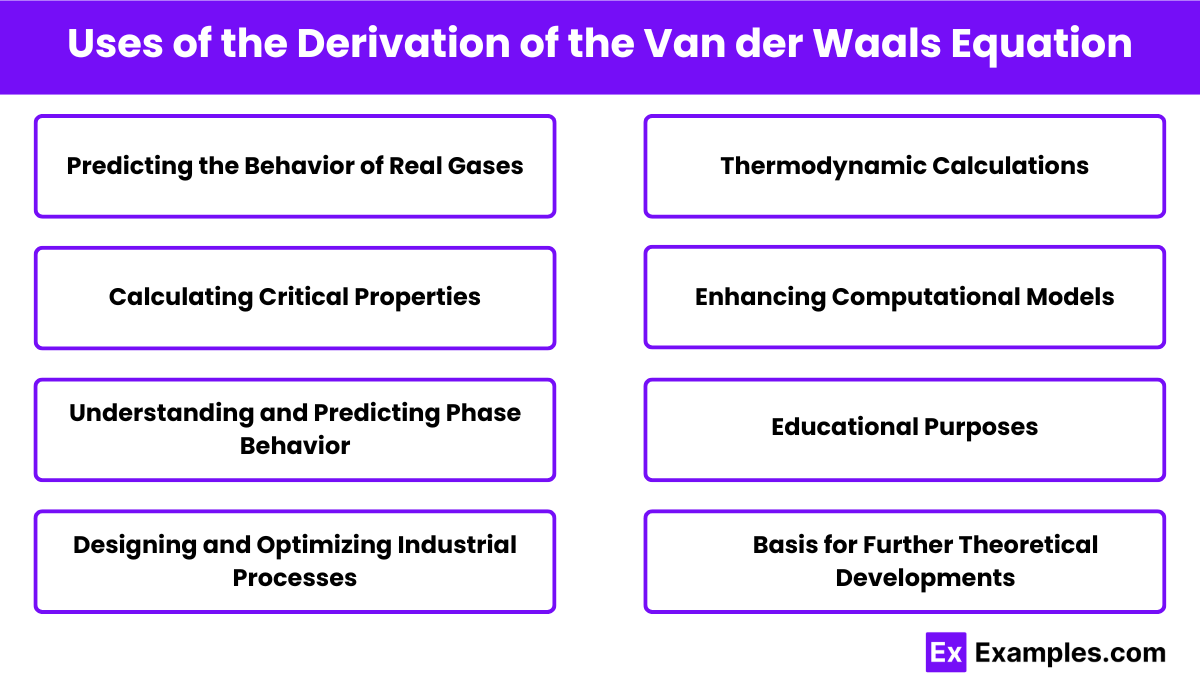
The derivation of the Van der Waals equation has several important uses in the field of physical chemistry and engineering. Here are some key applications:
The Van der Waals equation describes the behavior of real gases by accounting for molecular size and intermolecular forces.
In the Van der Waals equation, 𝑎a represents the magnitude of intermolecular forces, and 𝑏b accounts for the finite size of molecules.
The Van der Waals equation is important because it provides a more accurate description of the behavior of real gases compared to the Ideal Gas Law.
The Van der Waals equation is derived by modifying the Ideal Gas Law to include terms for molecular volume and intermolecular forces.
The Van der Waals equation improves upon the Ideal Gas Law by providing a more realistic model for gas behavior under non-ideal conditions.
The Van der Waals equation includes a term for molecular volume to account for the space occupied by gas molecules themselves.
The Van der Waals equation provides more accurate predictions of gas behavior under high-pressure and low-temperature conditions.
The constants a and b are determined experimentally for each specific gas.
In the Van der Waals equation, 𝑉ₘ represents the molar volume of the gas.
In the Van der Waals equation, R represents the universal gas constant.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
The Van der Waals equation is an adjustment of which ideal gas law equation?
PV = nRT
PV = \frac{nRT}{V - b}
P = \frac{nRT}{V - b} - \frac{a n^2}{V^2}
P = \frac{nRT}{V} - \frac{a}{V^2}
In the Van der Waals equation, what does the term \frac{a n^2}{V^2} represent?
Correction for the volume of the gas molecules
Correction for intermolecular forces
Correction for pressure
Correction for temperature
What does the term b in the Van der Waals equation represent?
The volume occupied by the gas molecules
The pressure correction factor
The temperature correction factor
The number of moles of gas
In the Van der Waals equation, which term corrects for the volume occupied by gas molecules?
\frac{a n^2}{V^2}
V - b
P
T
How does the Van der Waals equation differ from the ideal gas law in terms of volume correction?
It does not account for volume correction.
It uses the actual volume instead of the ideal volume.
It subtracts a volume term b from the total volume.
It adds a volume term b to the total volume.
Which constant in the Van der Waals equation is related to the strength of intermolecular attractions?
b
a
R
T
What effect does increasing the value of a have on the Van der Waals equation's prediction of pressure?
It decreases the predicted pressure.
It increases the predicted pressure.
It has no effect on the predicted pressure.
It makes the pressure prediction more accurate.
How does the Van der Waals equation account for the non-ideal behavior of gases at high pressures?
By increasing the volume term
By correcting the pressure term for intermolecular forces
By ignoring temperature effects
By assuming a constant volume
In the context of the Van der Waals equation, what does a higher value of b indicate?
Larger molecules with more volume
Smaller molecules with less volume
Higher temperature
Lower pressure
Which variable is adjusted by the Van der Waals equation to account for the volume occupied by gas molecules?
Pressure
Number of moles
Volume
Temperature
Before you leave, take our quick quiz to enhance your learning!

