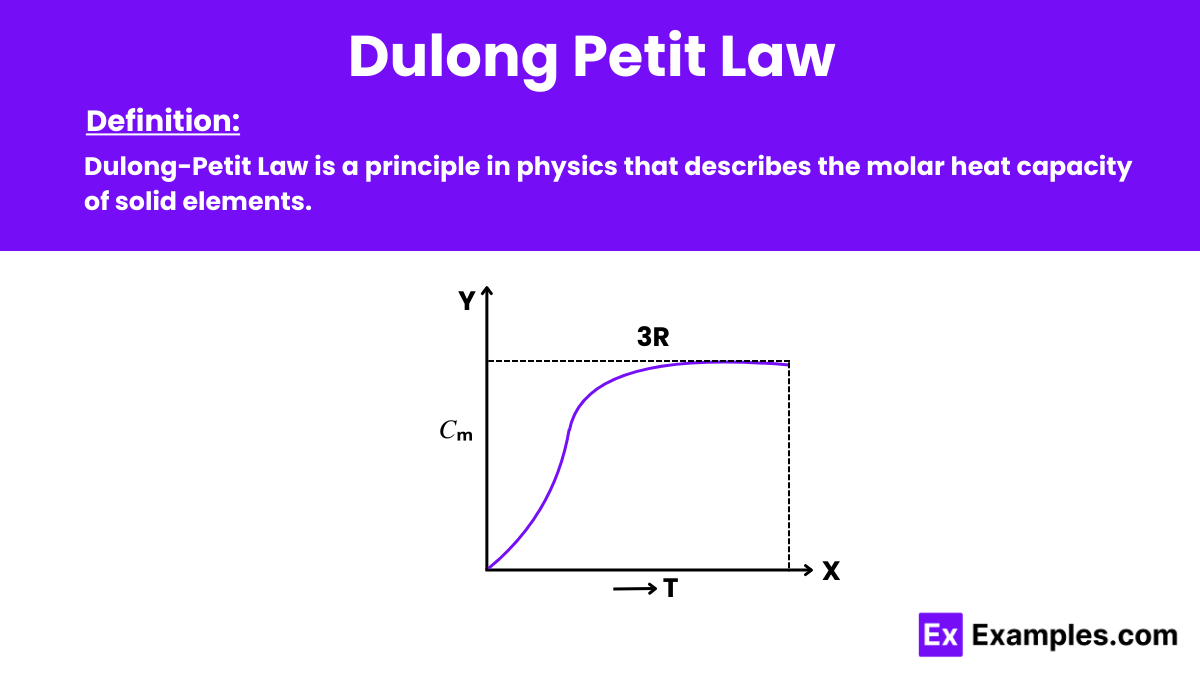
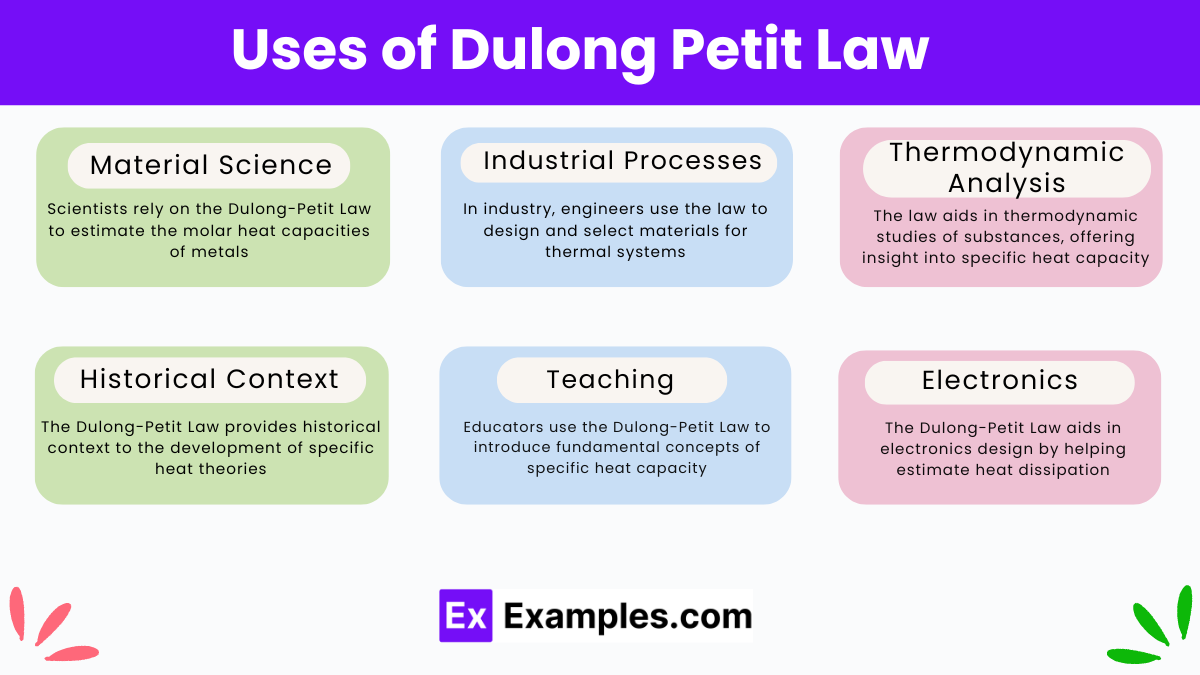
What does the Dulong-Petit Law state about the molar specific heat capacity of a solid element?
It is proportional to the atomic mass.
It is inversely proportional to the atomic mass.
It is approximately constant for most elements.
It varies significantly for different elements.