Which of the following is the unit of electric charge?
Newton
Joule
Coulomb
Watt

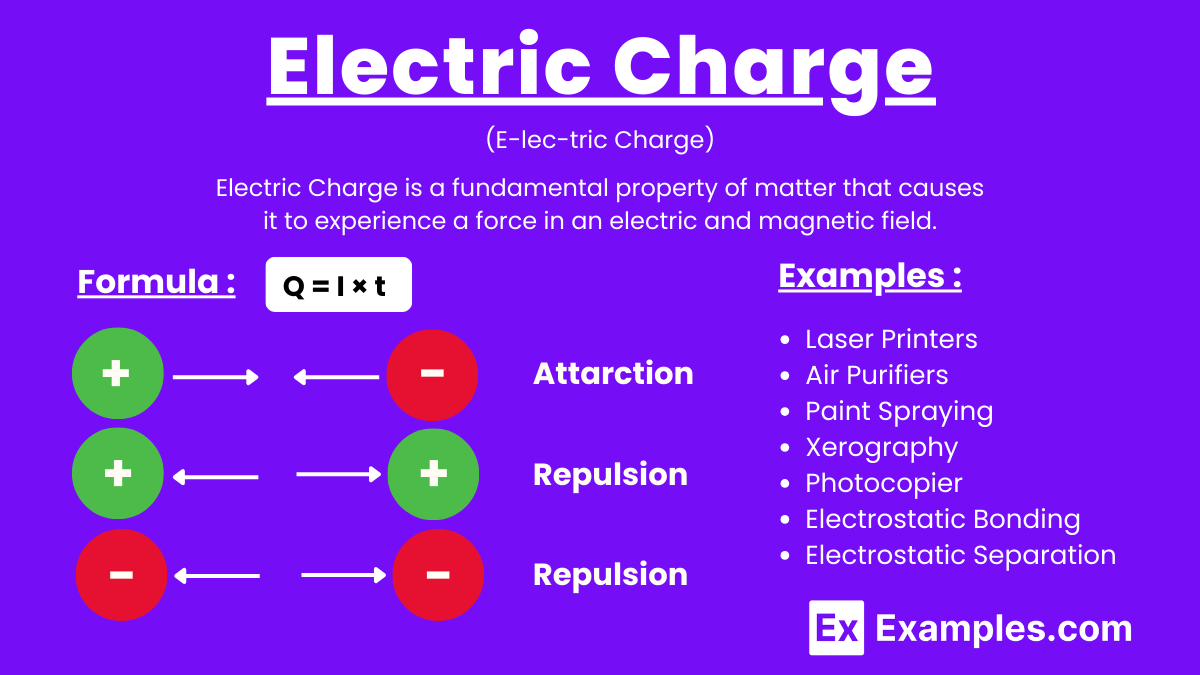
Electric charge is a fundamental property of matter that causes it to experience a force in an electromagnetic field. It comes in two types: positive and negative. The unit of electric charge in the International System of Units (SI) is the coulomb (C). Electric charge is quantized, with the elementary charge being 1.602×10⁻¹⁹ coulombs. The units of electric charge relate to the units of current, where one ampere (A) is one coulomb per second. Additionally, the electronvolt (eV) measures the Units of energy change when an electron moves through a potential difference of one volt.
Electric Charge is a fundamental property of matter that causes it to experience a force in an electric and magnetic field. It is carried by particles like protons and electrons, which have positive and negative charges, respectively. The SI unit of electric charge is the coulomb (C). Like charges repel, and opposite charges attract, following Coulomb’s law.
The formula for electric charge (Q) is derived from the relationship between current (I) and time (t):
Q: Electric charge in coulombs (C)
I: Electric current in amperes (A)
t: Time in seconds (s)
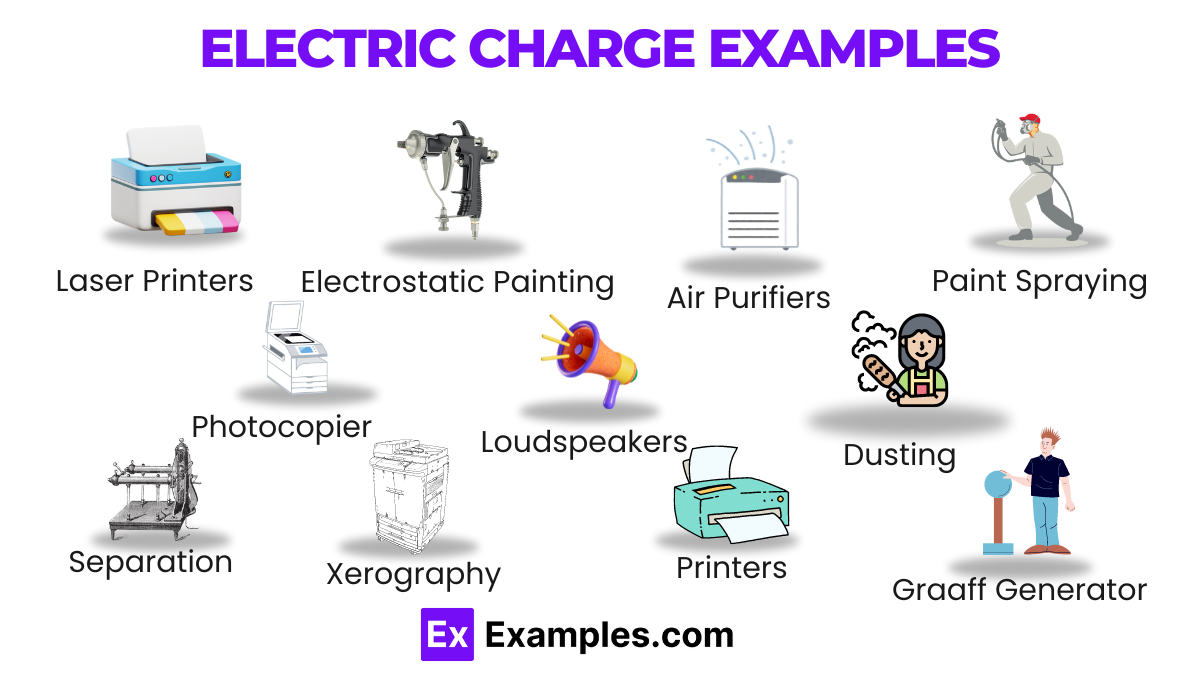
Electric charge is a fundamental property of matter that causes it to experience a force when placed in an electromagnetic field. There are two main types of electric charge: positive charge and negative charge. Understanding these types is crucial for studying electric fields, circuits, and various physical phenomena.
Positive charge, denoted by the symbol (+), is carried by protons. Protons are found in the nucleus of an atom. When an object has more protons than electrons, it is said to be positively charged. Common examples include:
Negative charge, denoted by the symbol (-), is carried by electrons. Electrons orbit the nucleus of an atom. When an object has more electrons than protons, it is negatively charged. Common examples include:
While not a type of electric charge, it’s important to note that objects can also be electrically neutral. This occurs when the number of protons equals the number of electrons, resulting in no net charge. Examples include:
| Characteristic | Positive Charge | Negative Charge |
|---|---|---|
| Carrier Particle | Protons | Electrons |
| Symbol | + | – |
| Behavior in Electric Field | Repel other positive charges, attract negative charges | Repel other negative charges, attract positive charges |
| Common Forms | Cations (e.g., Na, K) | Anions (e.g., Cl,O2) |
Charging is the process of giving an object an electric charge. There are three main methods of charging: friction, conduction, and induction. Each method works differently.
Charging by friction occurs when two objects are rubbed together, causing electrons to transfer. For example, rubbing a balloon on your hair transfers electrons from your hair to the balloon, making your hair positively charged and the balloon negatively charged. Similarly, rubbing a glass rod with silk transfers electrons to the silk, making the rod positively charged and the silk negatively charged.
Charging by conduction happens when a charged object touches a neutral object, transferring electrons. For instance, touching a charged metal rod to a neutral metal sphere transfers electrons to the sphere, charging it. Similarly, a charged balloon touching a wall transfers electrons, charging the wall.
Charging by induction occurs without direct contact. Bringing a charged balloon near a metal sphere repels electrons in the sphere, causing one side to be positively charged and the other negatively charged. Holding a charged rod near a grounded metal object also causes a separation of charges without direct contact.
Electric charge is a fundamental property of matter, and it comes with several key properties:
Like charges repel each other, while opposite charges attract. This fundamental principle is described by Coulomb’s law.
Coulomb’s law states that the force between two charges is directly proportional to the product of their magnitudes and inversely proportional to the square of the distance between them.
The unit of electric charge is the coulomb (C). One coulomb is equivalent to the charge of approximately 6.242 x 10¹⁸ electrons.
The charge of an electron is approximately -1.602 x 10⁻¹⁹ coulombs. This is considered a fundamental unit of charge.
The charge of a proton is approximately +1.602 x 10⁻¹⁹ coulombs, equal in magnitude but opposite in sign to the charge of an electron.
Materials become electrically charged through friction, conduction, or induction, which transfer or rearrange electrons between objects.
Static electricity is the result of an imbalance of charges on the surface of materials, often caused by friction.
An electric field is a region around a charged particle where other charges experience a force. It is represented by field lines pointing away from positive charges and toward negative charges.
The principle of charge conservation states that the total electric charge in an isolated system remains constant, regardless of any changes within the system.
Electric potential energy is the energy stored in a system of charges due to their positions relative to each other.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which of the following is the unit of electric charge?
Newton
Joule
Coulomb
Watt
What type of charge do protons possess?
Negative
Positive
Neutral
Variable
Which law describes the force between two point charges?
Ohm's Law
Coulomb's Law
Faraday's Law
Ampere's Law
What happens when two objects with the same type of charge come close to each other?
They attract
They repel
They neutralize
No effect
Which subatomic particle is responsible for electricity?
Proton
Neutron
Electron
Photon
What is the charge of a proton?
-1
+1
0
+2
How is static electricity typically generated?
By heating an object
By rubbing two objects together
By applying pressure
By shining light
What is the principle of conservation of charge?
Charge can be created and destroyed
Charge can only be created
Charge is conserved and cannot be created or destroyed
Charge is always neutral
Which material is a good conductor of electricity?
Rubber
Wood
Copper
Plastic
What device is used to detect the presence of electric charge?
Ammeter
Voltmeter
Electroscope
Galvanometer
Before you leave, take our quick quiz to enhance your learning!

