What is an electronvolt?
A unit of charge
A unit of current
A unit of energy
A unit of voltage
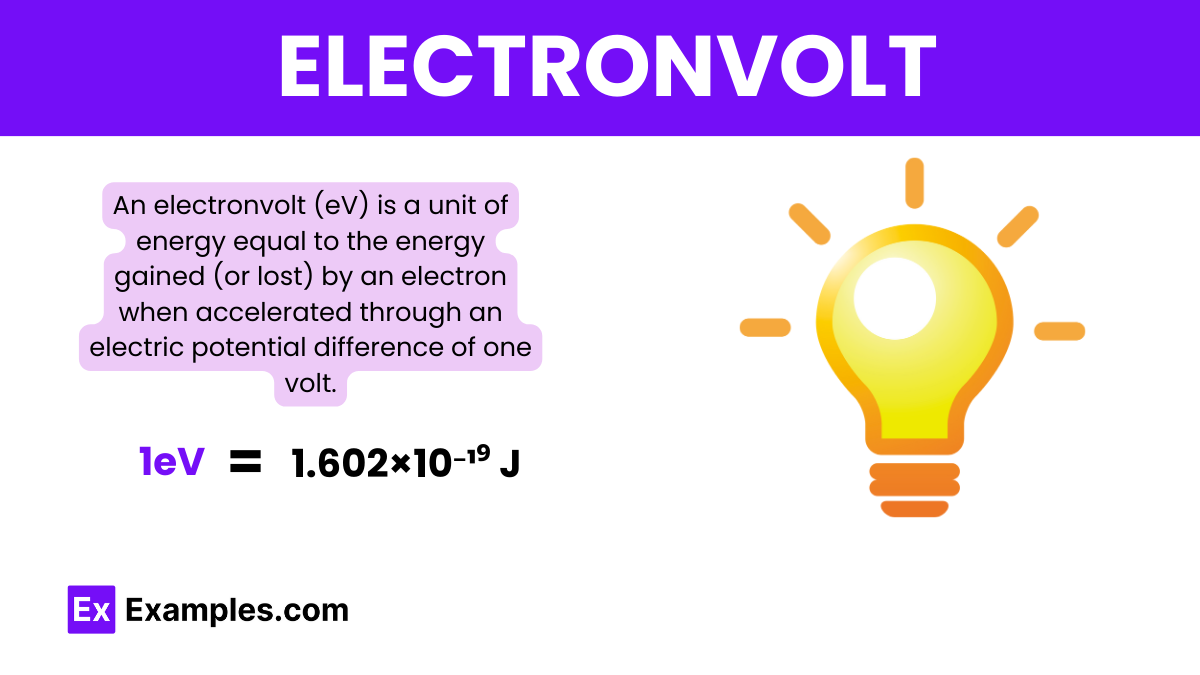
1eV = 1.602×10⁻¹⁹ J
1 electronvolt (eV) is equivalent to 1.602 × 10⁻¹⁹ joules, representing the energy gained by an electron when it moves across an electric potential difference of one volt.
The fundamental formula to calculate work or energy in Electronvolt (eV) is given by:
Kinetic Energy: The kinetic energy (KE) of a particle with a velocity (v) can be calculated using the formula: KEₑv=1/2 mv² Where:
Potential Energy:The potential energy (PE) of a particle in an electric field with a voltage (v) can be calculated using the formula: PEₑv=qV Where:
Electrical Energy:
The electrical energy (EE) associated with moving a charge q through a voltage V can be calculated using the formula: EEₑv=qV Where:
| Prefix | Symbol | Factor | Example |
|---|---|---|---|
| Exa- | EeV | 10¹⁸ | 1 EeV=10¹⁸ eV |
| Peta- | PeV | 10¹⁵ | 1 PeV=10¹⁵ eV |
| Tera- | TeV | 10¹² | 1 TeV=10¹²eV |
| Giga- | GeV | 10⁹ | 1 GeV=10⁹ eV |
| Mega- | MeV | 10⁶ | 1 MeV=10⁶ eV |
| Kilo- | keV | 10³ | 1 keV=10³ eV |
| Micro- | μeV | 10⁻⁶ | μeV=10⁻⁶ eV |
| Nano- | neV | 10⁻⁹ | 1 neV=10⁻⁹ eV |
| Pico- | peV | 10⁻¹² | 1 peV=10⁻¹² eV |
| Femto- | feV | 10⁻¹⁵ | 1 feV=10⁻¹⁵ eV |
| Atto- | aeV | 10⁻¹⁸ | 1 aeV=10⁻¹⁸ eV |
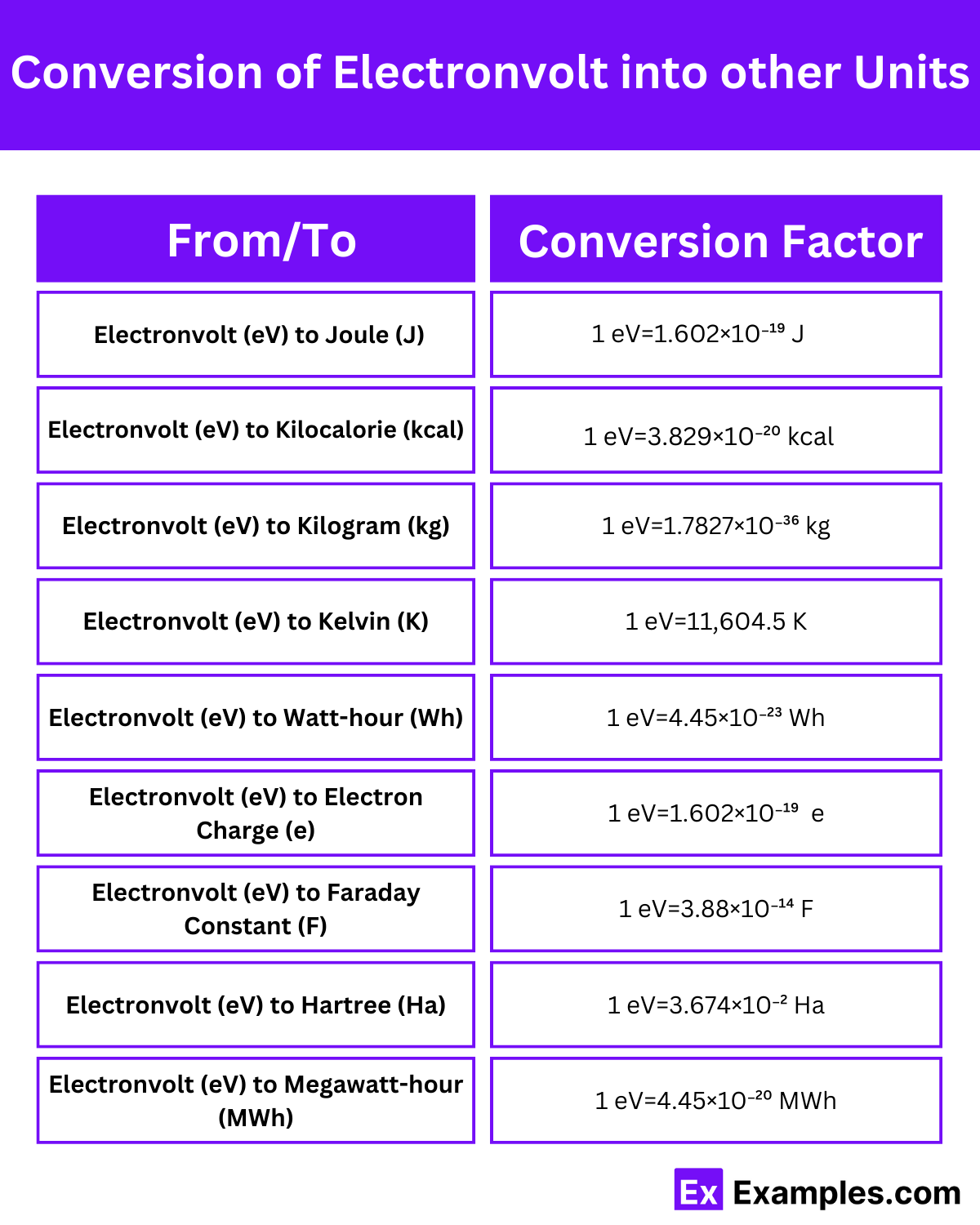
| From/To | Conversion Factor | Example Conversion |
|---|---|---|
| Electronvolt (eV) to Joule (J) | 1 eV=1.602×10⁻¹⁹ J | 1 eV=1.602×10⁻¹⁹ J |
| Electronvolt (eV) to Kilocalorie (kcal) | 1 eV=3.829×10⁻²⁰ kcal | 1 eV=3.829×10⁻²⁰ kcal |
| Electronvolt (eV) to Kilogram (kg) | 1 eV=1.7827×10⁻³⁶ kg | 1 eV=1.7827×10⁻³⁶ kg |
| Electronvolt (eV) to Kelvin (K) | 1 eV=11,604.5 K | 1 eV=11,604.5 K |
| Electronvolt (eV) to Watt-hour (Wh) | 1 eV=4.45×10⁻²³ Wh | 1 eV=4.45×10⁻²³ Wh |
| Electronvolt (eV) to Electron Charge (e) | 1 eV=1.602×10⁻¹⁹ e | 1 eV=1.602×10⁻¹⁹ e |
| Electronvolt (eV) to Faraday Constant (F) | 1 eV=3.88×10⁻¹⁴ F | 1 eV=3.88×10⁻¹⁴ F |
| Electronvolt (eV) to Hartree (Ha) | 1 eV=3.674×10⁻² Ha | 1 eV=3.674×10⁻² Ha |
| Electronvolt (eV) to Megawatt-hour (MWh) | 1 eV=4.45×10⁻²⁰ MWh | 1 eV=4.45×10⁻²⁰ MWh |
Electronvolts (eV) can be converted into joules (J), which are the SI unit of energy, using a specific conversion factor.
Electronvolts (eV) can also be converted into kilocalories (kcal), which are commonly used in nutrition to measure food energy content.
Electronvolts (eV) can be converted into kilograms (kg), which are the SI unit of mass, using a specific conversion factor.
Electronvolts (eV) can also be converted into kelvins (K), which are the SI unit of temperature.
Electronvolts (eV) can be converted into watt-hours (Wh), which are a unit of energy commonly used in electricity billing and power generation.
Electronvolts (eV) can be converted into electron charges (e), which represent the elementary charge of an electron.
Electronvolts (eV) can be converted into the Faraday constant (F), which relates the amount of electric charge in a mole of electrons to the energy required to move it.
Electronvolts (eV) can be converted into Hartrees (Ha), which are a unit of energy used in atomic and molecular physics.
Electronvolts (eV) can also be converted into megawatt-hours (MWh), which are a unit of energy commonly used in the electricity industry.
One practical application example of electronvolts (eV) is in the field of semiconductor physics, particularly in band theory. In semiconductor devices like transistors and diodes, electrons are excited from the valence band to the conduction band by absorbing energy in the form of electronvolts. This energy level difference, measured in electronvolts, determines the conductivity and behavior of the semiconductor material. For instance, in light-emitting diodes (LEDs), the energy difference between the valence and conduction bands corresponds to the energy of emitted photons, which determines the color of light produced by the LED.
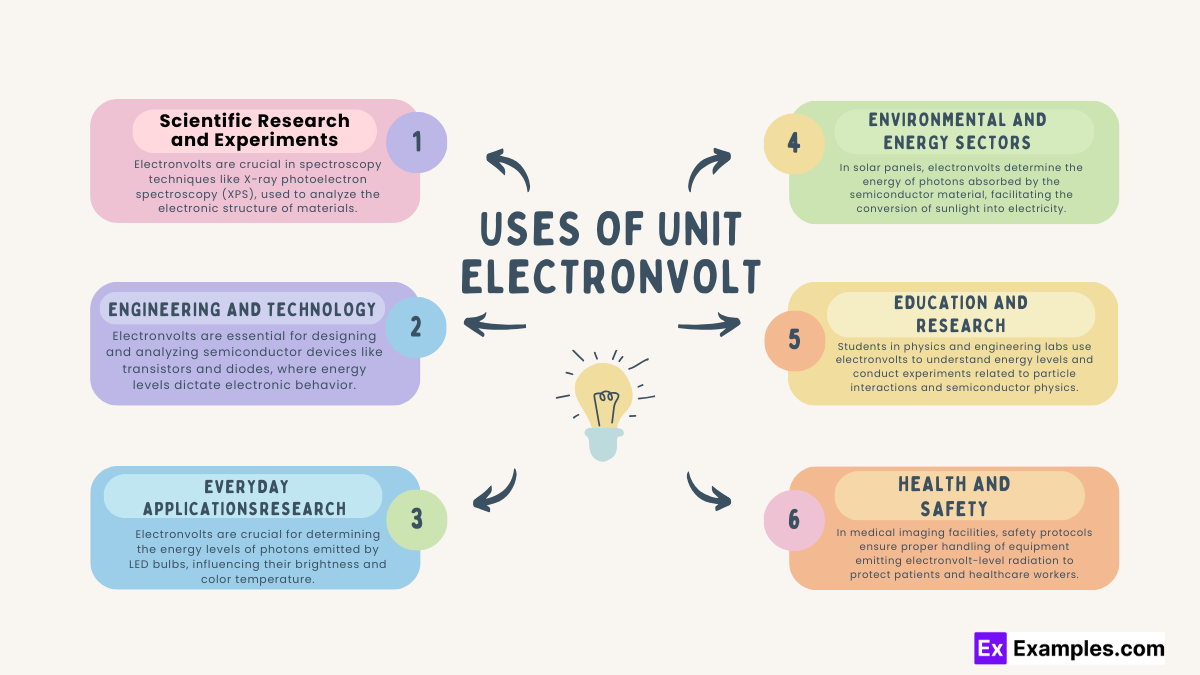
Material Science: Electronvolts are crucial in spectroscopy techniques like X-ray photoelectron spectroscopy (XPS).
Nuclear Physics: Studies involving nuclear reactions and decay often rely on electronvolts to measure energy levels and transitions in atomic nuclei.
Semiconductor Devices: Electronvolts are essential for designing and analyzing semiconductor devices like transistors and diodes, where energy levels dictate electronic behavior.
Solar Energy: In photovoltaic cells, electronvolts determine the energy of photons absorbed by the semiconductor material, influencing solar cell efficiency.
Medical Imaging: Electronvolts play a role in medical imaging technologies like X-ray machines and computed tomography (CT) scanners, where energy levels affect image quality and diagnostic accuracy.
Consumer Electronics: In devices like smartphones and laptops, electronvolts influence the performance of semiconductor components such as microprocessors and memory chips.
Lighting Technology: Electronvolts are crucial for determining the energy levels of photons emitted by LED bulbs, influencing their brightness and color temperature.
Battery Technology: Electronvolts are involved in the charging and discharging processes of batteries, impacting the energy storage capacity and efficiency of portable electronic devices.
Renewable Energy: In solar panels, electronvolts determine the energy of photons absorbed by the semiconductor material, facilitating the conversion of sunlight into electricity.
Energy Efficiency: Electronvolts play a role in optimizing energy efficiency in various technologies, from lighting systems to electric vehicles, by understanding and controlling energy levels in electronic components.
Particle Physics: High-energy particle accelerators, such as the Large Hadron Collider (LHC), use electronvolts to describe the energy of particles and collisions.
Laboratory Experiments: Students in physics and engineering labs use electronvolts to understand energy levels and conduct experiments related to particle interactions and semiconductor physics.
Radiation Safety: In medical imaging facilities, safety protocols ensure proper handling of equipment emitting electronvolt-level radiation to protect patients and healthcare workers.
Occupational Exposure Limits: Regulatory agencies establish limits for electronvolt radiation exposure in workplaces to safeguard workers in industries like nuclear power and radiography.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is an electronvolt?
A unit of charge
A unit of current
A unit of energy
A unit of voltage
How many joules are there in one electronvolt?
1.6 x 10⁻¹⁹ J
1.6 x 10⁻¹⁸ J
1.6 x 10⁻²⁰ J
1.6 x 10⁻¹⁷ J
What physical quantity is typically measured in electronvolts?
Mass
Length
Energy
Time
If an electron is accelerated through a potential difference of 5 volts, what is its energy in electronvolts?
1 eV
5 eV
10 eV
0.2 eV
Which of the following best describes the relationship between electronvolts and joules?
They are units of different physical quantities
They are both units of energy
Joules measure charge, and electronvolts measure energy
Electronvolts are units of voltage, and joules are units of energy
How much energy does an electron gain when it is accelerated through a potential difference of 1 volt?
1 joule
1 coulomb
1 electronvolt
1 watt
Which of the following conversions is correct for energy?
1 eV = 1.6 x 10⁻¹⁷ J
1 eV = 1.6 x 10⁻²⁰ J
1 eV = 1.6 x 10⁻¹⁹ J
1 eV = 1.6 x 10⁻¹⁸ J
What is the significance of the electronvolt in particle physics?
It measures the charge of particles
It measures the velocity of particles
It measures the energy of particles
It measures the mass of particles
How much energy in electronvolts does a proton gain when accelerated through a potential difference of 3 volts?
1 eV
3 eV
4 eV
5 eV
Which of the following best describes 1 MeV?
10⁶ eV
10³ eV
10⁹ eV
10¹² eV
Before you leave, take our quick quiz to enhance your learning!

