What is the formula for the energy levels of an electron in a hydrogen atom?
Eₙ = -13.6/n² eV
Eₙ = -13.6n² eV
Eₙ = 13.6/n eV
Eₙ = 13.6n eV

Energy levels in physics refer to the distinct quantities of energy that an electron in an atom can possess. These levels are quantized, meaning electrons can only occupy specific energy states, not values in between. The concept of energy levels is pivotal in understanding atomic structure and electron behavior.
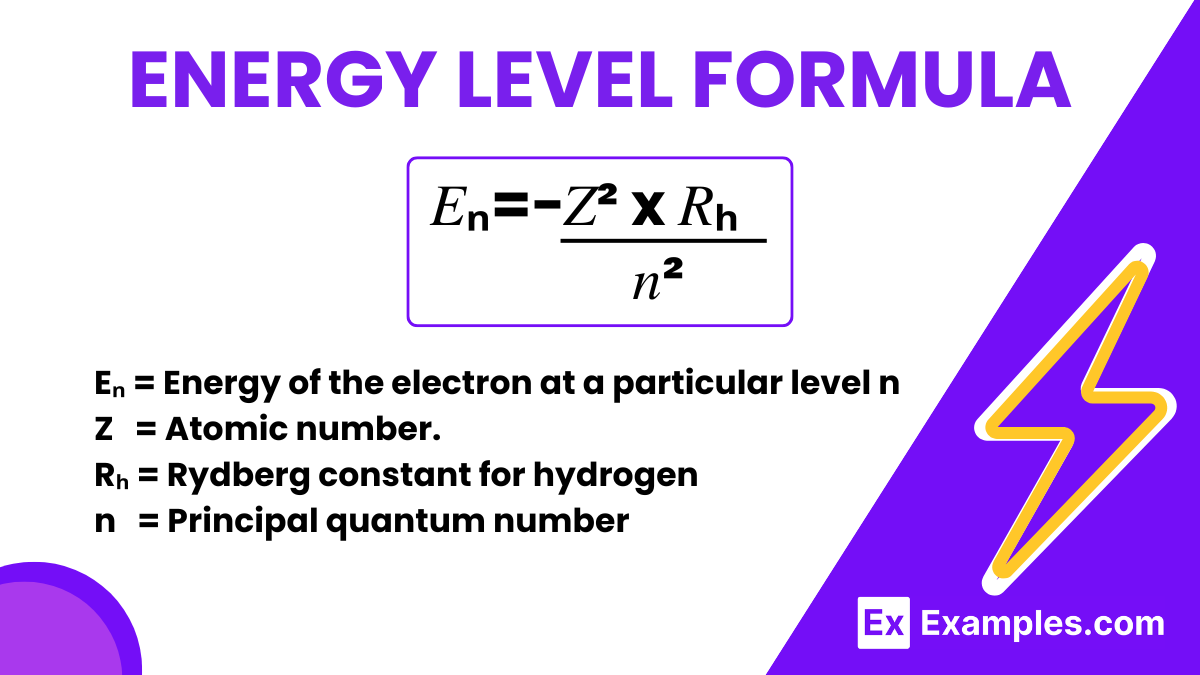
The energy level formula, particularly for hydrogen-like atoms, is given by:
This formula was derived from the Bohr model of the atom, proposed by Niels Bohr in 1913. Bohr introduced the idea that electrons orbit the nucleus in specific stable orbits without radiating energy, and these orbits correspond to certain quantized energy levels. Energy is only absorbed or emitted by an electron when it moves from one energy level to another, not while it remains in a stable orbit.
The minus sign in the formula indicates that the energy levels are bound states (the electron is bound to the nucleus), and the energy level decreases (becomes more negative) as the electron gets closer to the nucleus. As n increases, the electron’s energy becomes less negative, indicating it is less tightly bound and closer to escaping the atom’s influence.
This simple yet profound explanation helps us understand atomic spectra, chemical bonding, and other fundamental aspects of physics and chemistry. Niels Bohr’s contributions have been foundational in both theoretical and applied physics.
Problem: Calculate the energy of an electron in the third energy level (𝑛=3) of a hydrogen atom (𝑍=1).
Solution: Using the formula:
E₃ = – (1² x 2.18 x 10⁻¹⁸ J ) / 3² = – (2.18 x 10⁻¹⁸ J ) / 9 = -0.2422 x 10⁻¹⁸ J
E₃ = -2.422 x 10⁻¹⁹ J
The energy of an electron in the third level of a hydrogen atom is approximately −2.422×10⁻¹⁹ J
Problem: Determine the energy released when an electron transitions from the fifth energy level (n=5) to the second energy level (n=2) in a hydrogen atom (Z=1).
Solution: First, calculate the energy at n=5 andn=2:
E₅ = – (1² x 2.18 x 10⁻¹⁸ J ) / 5² = – 2.18 x 10⁻¹⁸ J / 25 = – 0.0872 x 10⁻¹⁸ J
E₂ = – (1² x 2.18 x 10⁻¹⁸ J ) /2² = – 2.18 x 10⁻¹⁸ J / 4 = -0.545 x 10⁻¹⁸ J
The energy released during the transition:
Δ𝐸=E₂ −E₅ = (− 0.545 x 10⁻¹⁸ J ) – (-0.0872 x 10⁻¹⁸ J)
Δ𝐸 = -0.545 x 10⁻¹⁸ J + 0.0872 x 10⁻¹⁸ J
Δ𝐸 = -0.4578 x 10⁻¹⁸ J
The energy released is approximately −0.4578×10−18 J, which signifies energy emission as the electron moves to a lower energy state.
Problem: Calculate and compare the energy of an electron in the first energy level (𝑛=1) of hydrogen (𝑍=1) and helium-ion (𝑍=2).
Solution: For hydrogen (𝑍=1):
𝐸₁ᴴ = − (1² x 2.18×10⁻¹⁸ J ) / 1²=−2.18×10⁻¹⁸ J
For helium-ion (Z=2):
𝐸₁ʰᵉ⁺=− (2²⋅2.18 × 10⁻¹⁸ J )/ 12 =− (4 x 2.18 × 10⁻¹⁸ J) / 1=−8.72×10⁻¹⁸ J
Use the energy level formula: 𝐸ₙ=−𝑍²⋅𝑅ₕ / 𝑛², where Z is the atomic number and n the principal quantum number.
The famous energy formula is 𝐸=𝑚𝑐², introduced by Einstein, relating energy (E), mass (m), and the speed of light (c).
The SI unit of energy is the joule (J), measuring energy, work, or heat in scientific contexts.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the formula for the energy levels of an electron in a hydrogen atom?
Eₙ = -13.6/n² eV
Eₙ = -13.6n² eV
Eₙ = 13.6/n eV
Eₙ = 13.6n eV
Which constant is used in the energy level formula of a hydrogen atom?
Planck's constant
Rydberg constant
Boltzmann constant
Avogadro's constant
If the principal quantum number n increases, what happens to the energy level of an electron in a hydrogen atom?
It decreases
It increases
It remains the same
It becomes zero
What is the energy of an electron in the n = 1 energy level of a hydrogen atom?
-0.85 eV
-1.51 eV
-13.6 eV
-3.4 eV
What happens to the spacing between energy levels as n increases in a hydrogen atom?
It decreases
It increases
It remains the same
It becomes zero
What is the energy of an electron in the n = 2 energy level of a hydrogen atom?
-13.6 eV
-3.4 eV
-1.51 eV
-0.85 eV
What is the ionization energy of a hydrogen atom?
0 eV
3.4 eV
-13.6 eV
13.6 eV
How does the energy of an electron in a hydrogen atom change as it moves to a higher energy level?
It becomes more negative
It becomes less negative
It remains constant
It becomes zero
What is the formula for the energy of a photon emitted when an electron transitions between two energy levels?
E = hν
E = mc²
E = qV
E = Fd
Which quantum number determines the energy level of an electron in a hydrogen atom?
Azimuthal quantum number
Magnetic quantum number
Principal quantum number
Spin quantum number
Before you leave, take our quick quiz to enhance your learning!

