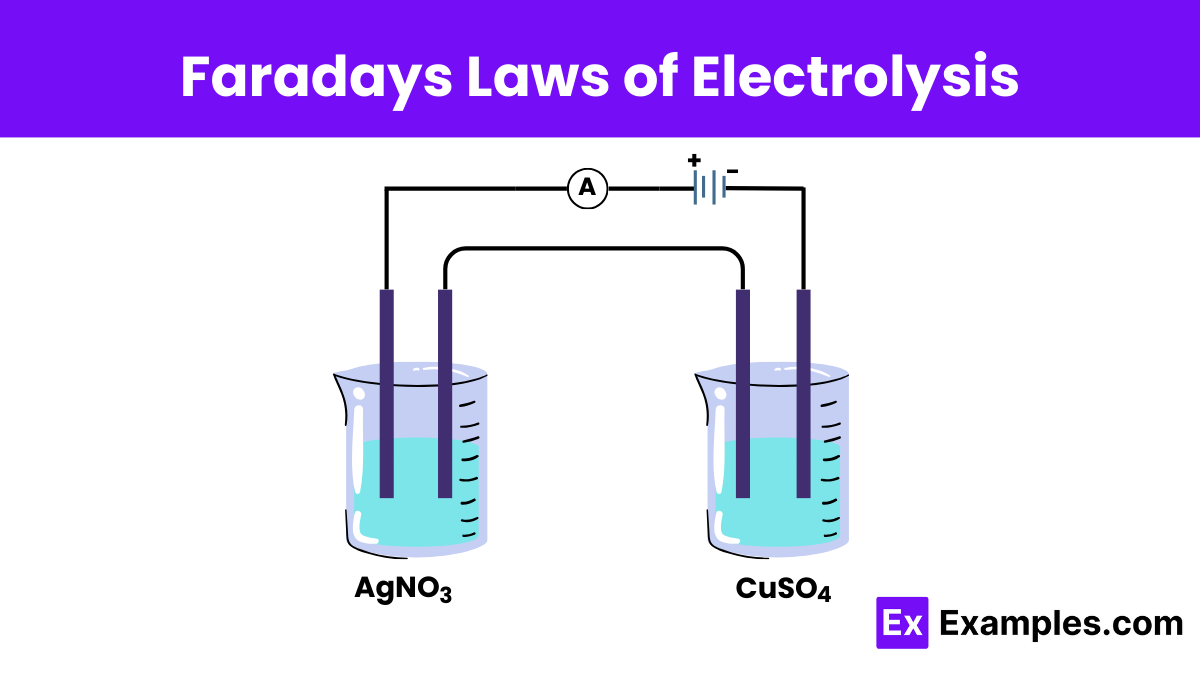
What does Faraday's First Law of Electrolysis state?
The mass of a substance produced at an electrode is directly proportional to the quantity of electricity.
The mass of a substance produced at an electrode is inversely proportional to the quantity of electricity.
The mass of a substance produced at an electrode is directly proportional to the electrode potential.
The mass of a substance produced at an electrode is inversely proportional to the electrode potential.