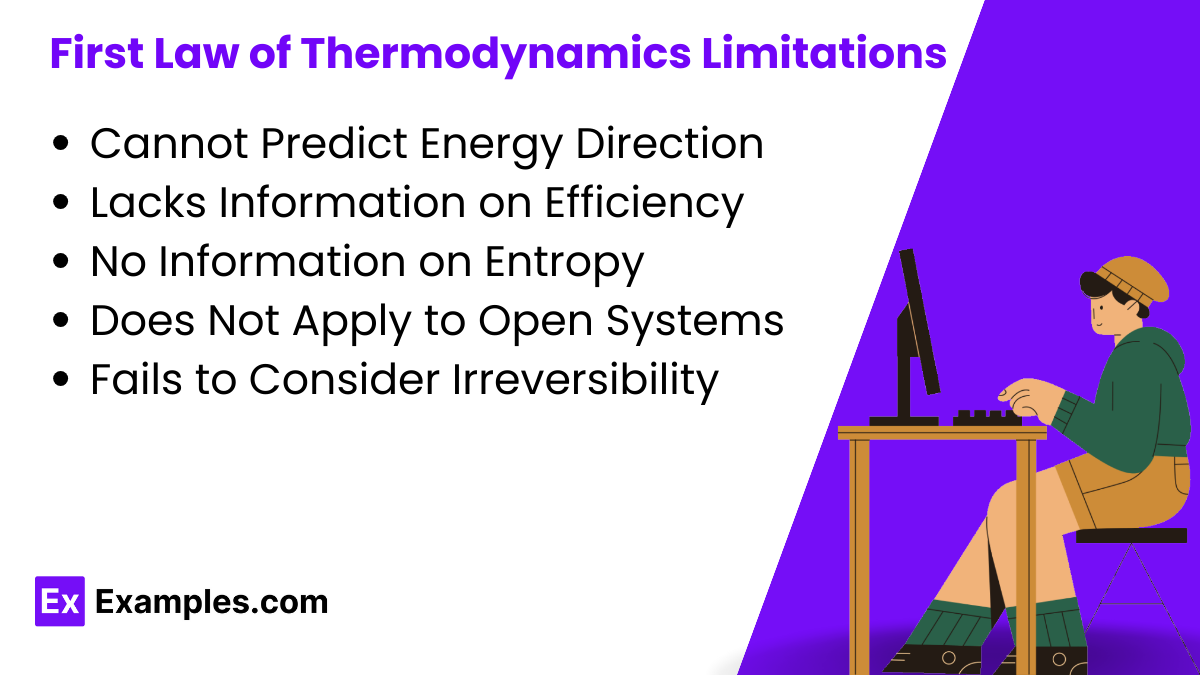
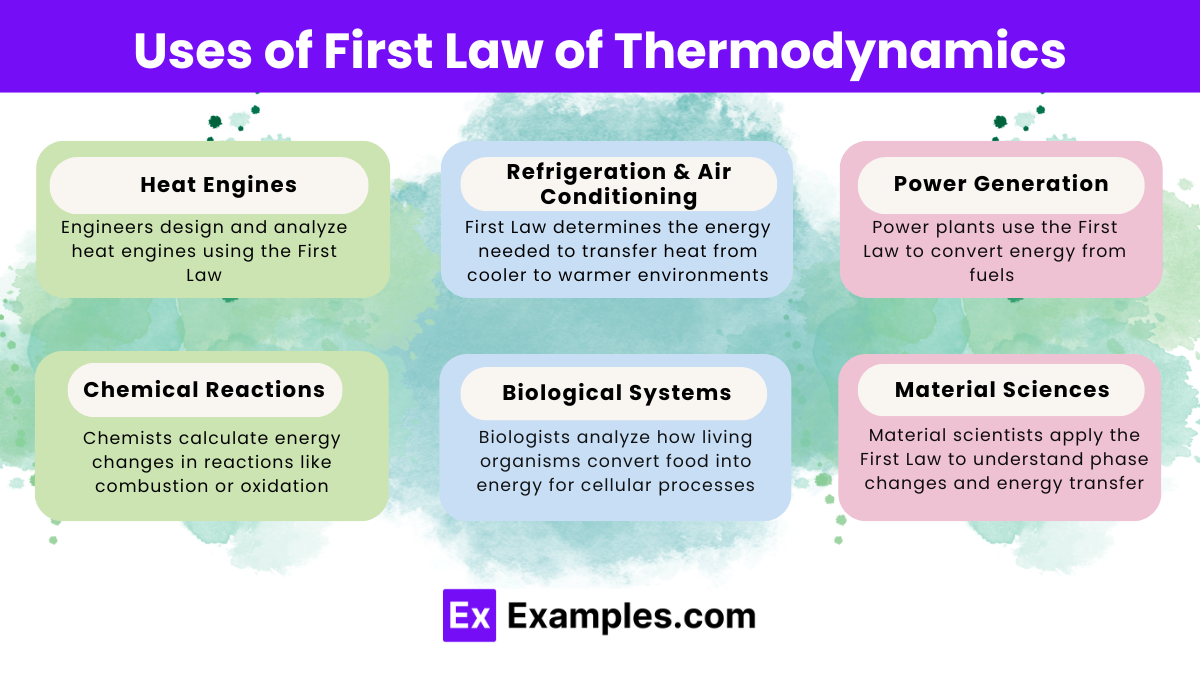
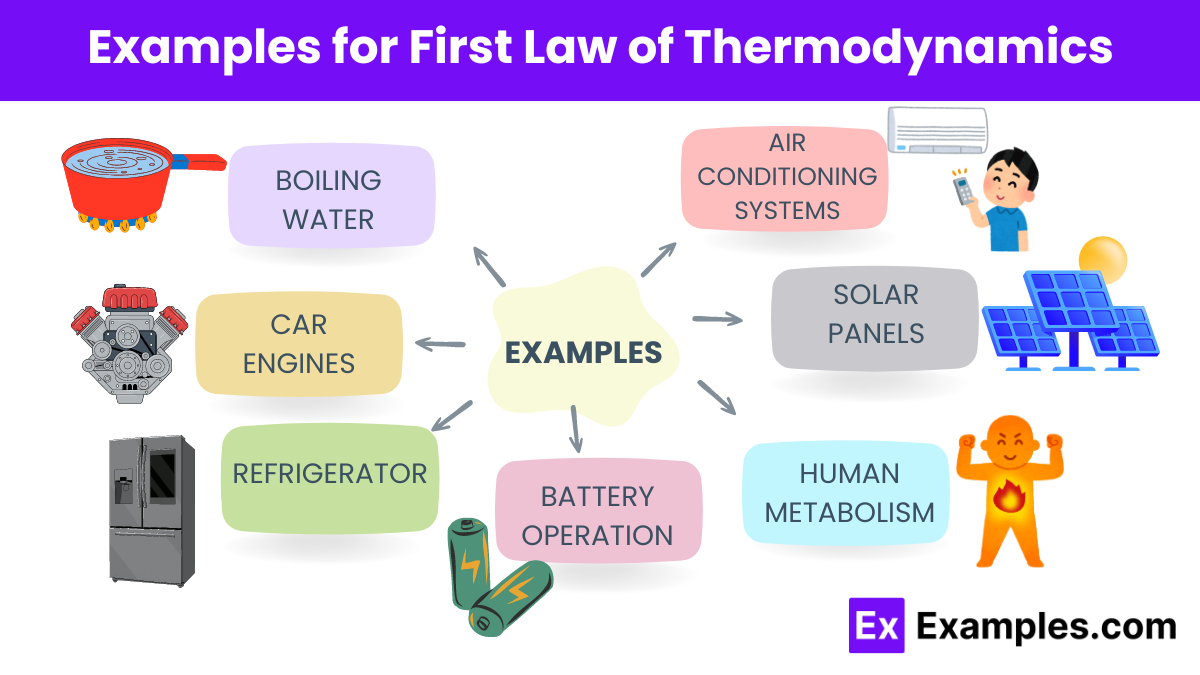
What does the First Law of Thermodynamics state?
Energy cannot be created or destroyed, only transferred or changed in form
Entropy of an isolated system always increases
Heat flows spontaneously from hot to cold bodies
Energy is always conserved in a closed system