What is the primary factor that determines the pressure in a fluid?
The density of the fluid
The volume of the fluid
The shape of the container
The temperature of the fluid

Fluids are substances that flow and take the shape of their container due to their inability to resist shear stress. This includes both liquids and gases, which deform continuously under an applied force. In a fluid, molecules move freely past one another, lacking a fixed shape. Fluids are characterized by properties such as density, viscosity, and pressure, which affect their behavior. The Laws of Fluid Dynamics describe fluid movement, including the continuity equation and Bernoulli’s principle. The US fluid ounce measures liquid volume, and Newton’s Law of Viscosity defines the relationship between shear stress and velocity gradient in a fluid.
Fluids are substances that can flow and take the shape of their container, including both liquids and gases. They deform under applied stress, allowing movement and adaptation to surroundings. Fluids are essential in natural and industrial processes, making them a key concept in physics and engineering.
Fluids can be classified into four main types based on their flow characteristics:
Steady Fluids: Steady fluids are those whose properties at any given point do not change over time. An example of this is water flowing at a constant rate through a pipe.
Unsteady Fluids: Unsteady fluids have properties that vary with time at any given point, such as wind speed fluctuating over time.
Compressible Fluids: Compressible fluids are those whose density changes significantly with pressure, typically gases. These fluids have a Mach Number between 0.3 and 1, with air at high speeds being a common example.
Incompressible Fluids: Incompressible fluids, usually liquids, maintain a relatively constant density regardless of pressure changes. Their Mach Number is less than 0.3, with water under normal conditions being a prime example.
Viscous Fluids: Viscous fluids exhibit significant resistance to flow due to their thickness or internal friction. Examples include shampoo and motor oil.
Non-Viscous Fluids: Non-viscous fluids have negligible resistance to flow and flow with minimal internal friction. Superfluid liquid helium is an example of a non-viscous fluid.
Rotational Fluids: Rotational fluids are those in which the fluid elements exhibit rotational motion, meaning the angle between intersecting lines of the fluid element changes as it moves. Turbulent water flow is an example of a rotational fluid.
Irrotational Fluids: Irrotational fluids do not exhibit rotational motion, allowing the fluid to move without changing the angles between boundary lines. Ideal fluid flow in theoretical models is an example of irrotational fluid.
Fluid Statics: Fluid statics is the study of fluids at rest, focusing on the pressure exerted by fluids on objects, such as hydrostatic pressure in a tank.
Fluid Dynamics: Fluid dynamics is the study of fluids in motion, encompassing fields like aerodynamics and hydrodynamics, with examples including airflow over an airplane wing and water flow in rivers.
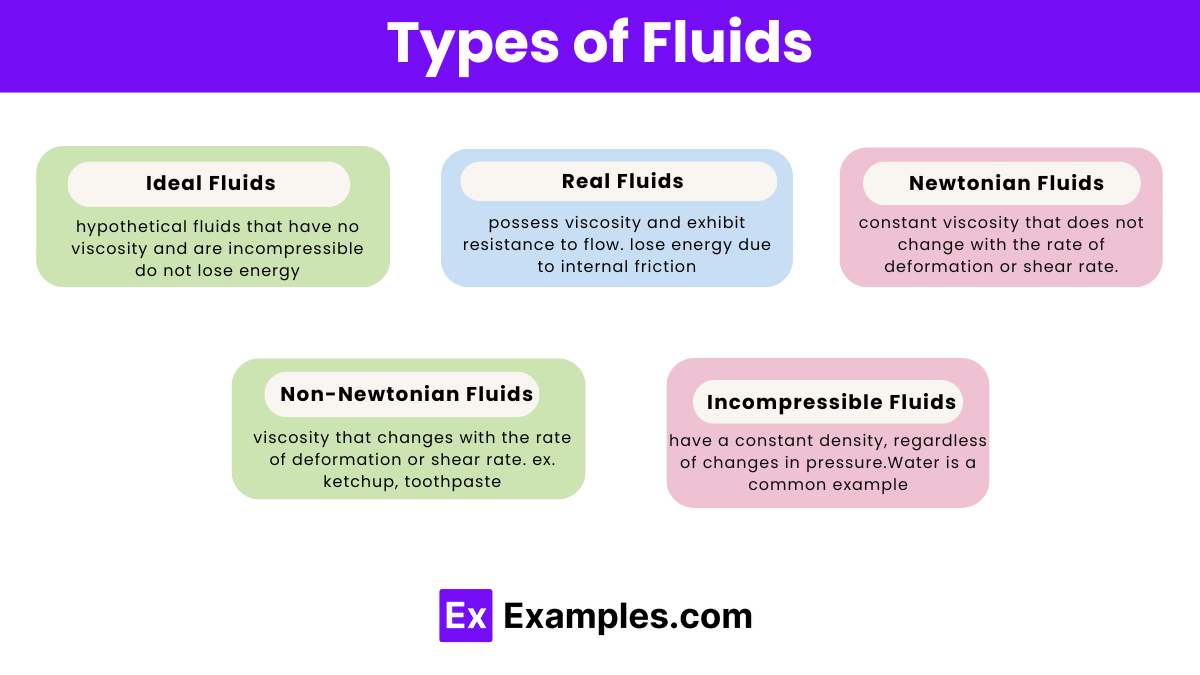
Ideal fluids are hypothetical fluids that have no viscosity and are incompressible. These fluids exhibit no internal resistance to flow and do not lose energy as they move. Although no real fluid perfectly matches these conditions, ideal fluids are primarily used in theoretical models to simplify calculations and understand fundamental fluid dynamics principles.
Real fluids possess viscosity and exhibit resistance to flow. Unlike ideal fluids, they lose energy due to internal friction as they move. Most practical fluids, such as water, air, and oil, fall into this category. Real fluids are essential in studying fluid mechanics in real-world applications.
Newtonian fluids have a constant viscosity that does not change with the rate of deformation or shear rate. The flow behavior of these fluids follows Newton’s law of viscosity, where the shear stress is directly proportional to the shear rate. Examples of Newtonian fluids include water, air, and thin motor oil. These fluids are commonly encountered in daily life and industrial processes.
Non-Newtonian fluids have a viscosity that changes with the rate of deformation or shear rate. These fluids do not follow Newton’s law of viscosity and can exhibit various flow behaviors, such as shear thinning, shear thickening, or viscoelasticity. Examples of non-Newtonian fluids include ketchup, toothpaste, and cornstarch mixed with water. These fluids are often encountered in household and industrial applications where complex flow behavior is observed.
Incompressible fluids have a constant density, regardless of changes in pressure. Most liquids are considered incompressible because their density does not significantly change under normal conditions. Water is a common example of an incompressible fluid. Incompressible fluids are crucial in hydraulic systems and fluid mechanics calculations where volume changes are negligible.
Compressible fluids have a density that changes significantly with pressure. Gases are typically compressible because their volume and density vary with pressure and temperature changes. Air is a common example of a compressible fluid. Understanding compressible fluids is essential in aerodynamics, gas dynamics, and high-speed fluid flow applications.
To help understand the properties of fluids, here are some practice problems. Each problem includes a detailed solution
Question: A cylindrical container with a radius of 5 cm and a height of 10 cm is filled with a liquid. The mass of the liquid is 2 kg. Calculate the density of the liquid.
Solution:
Answer: The density of the liquid is 2548 kg/m³.
Question: What is the pressure at a depth of 5 meters below the surface of a lake? (Assume the density of water is 1000 kg/m³ and acceleration due to gravity is 9.81 m/s²).
Solution:
Answer: The pressure at a depth of 5 meters is 49050 Pa (Pascals).
Liquids have a definite volume but no fixed shape, while gases have neither a definite volume nor a fixed shape.
Buoyancy is the upward force exerted by a fluid on an immersed object, allowing it to float or sink.
Fluid pressure is the force exerted per unit area by a fluid on a surface.
Pascal’s Principle states that a change in pressure applied to an enclosed fluid is transmitted equally throughout the fluid.
Bernoulli’s Principle states that an increase in fluid speed results in a decrease in pressure or potential energy.
The continuity equation states that the mass flow rate of a fluid remains constant from one cross-section of a pipe to another.
Laminar flow is smooth and orderly, while turbulent flow is chaotic and irregular.
Temperature can change the viscosity and density of a fluid, affecting its flow characteristics.
Surface tension is the cohesive force at the surface of a liquid, affecting phenomena like capillary action.
Archimedes’ Principle states that the buoyant force on an object is equal to the weight of the fluid displaced by the object.

Fluids are substances that flow and take the shape of their container due to their inability to resist shear stress. This includes both liquids and gases, which deform continuously under an applied force. In a fluid, molecules move freely past one another, lacking a fixed shape. Fluids are characterized by properties such as density, viscosity, and pressure, which affect their behavior. The Laws of Fluid Dynamics describe fluid movement, including the continuity equation and Bernoulli’s principle. The US fluid ounce measures liquid volume, and Newton’s Law of Viscosity defines the relationship between shear stress and velocity gradient in a fluid.
Fluids are substances that can flow and take the shape of their container, including both liquids and gases. They deform under applied stress, allowing movement and adaptation to surroundings. Fluids are essential in natural and industrial processes, making them a key concept in physics and engineering.
Water – Found in rivers, oceans, and lakes.
Oil – Used as a lubricant and fuel.
Milk – A nutrient-rich liquid produced by mammals.
Blood – Circulates through the bodies of animals, delivering nutrients and oxygen.
Honey – A thick, sweet liquid produced by bees.
Alcohol – Used in beverages and as a solvent.
Mercury – A heavy, metallic liquid at room temperature.
Juice – Extracted from fruits and vegetables.
Gasoline – A fuel used in internal combustion engines.
Lava – Molten rock expelled by a volcano during an eruption.
Syrup – A thick, sweet liquid used in cooking and as a topping.
Plasma – The fourth state of matter, consisting of ionized gases.
Tears – A clear liquid produced by the lacrimal glands in the eyes.
Seawater – Saltwater found in oceans and seas.
Fluids can be classified into four main types based on their flow characteristics:
Steady Fluids: Steady fluids are those whose properties at any given point do not change over time. An example of this is water flowing at a constant rate through a pipe.
Unsteady Fluids: Unsteady fluids have properties that vary with time at any given point, such as wind speed fluctuating over time.
Compressible Fluids: Compressible fluids are those whose density changes significantly with pressure, typically gases. These fluids have a Mach Number between 0.3 and 1, with air at high speeds being a common example.
Incompressible Fluids: Incompressible fluids, usually liquids, maintain a relatively constant density regardless of pressure changes. Their Mach Number is less than 0.3, with water under normal conditions being a prime example.
Viscous Fluids: Viscous fluids exhibit significant resistance to flow due to their thickness or internal friction. Examples include shampoo and motor oil.
Non-Viscous Fluids: Non-viscous fluids have negligible resistance to flow and flow with minimal internal friction. Superfluid liquid helium is an example of a non-viscous fluid.
Rotational Fluids: Rotational fluids are those in which the fluid elements exhibit rotational motion, meaning the angle between intersecting lines of the fluid element changes as it moves. Turbulent water flow is an example of a rotational fluid.
Irrotational Fluids: Irrotational fluids do not exhibit rotational motion, allowing the fluid to move without changing the angles between boundary lines. Ideal fluid flow in theoretical models is an example of irrotational fluid.
Fluid Statics: Fluid statics is the study of fluids at rest, focusing on the pressure exerted by fluids on objects, such as hydrostatic pressure in a tank.
Fluid Dynamics: Fluid dynamics is the study of fluids in motion, encompassing fields like aerodynamics and hydrodynamics, with examples including airflow over an airplane wing and water flow in rivers.

Ideal fluids are hypothetical fluids that have no viscosity and are incompressible. These fluids exhibit no internal resistance to flow and do not lose energy as they move. Although no real fluid perfectly matches these conditions, ideal fluids are primarily used in theoretical models to simplify calculations and understand fundamental fluid dynamics principles.
Real fluids possess viscosity and exhibit resistance to flow. Unlike ideal fluids, they lose energy due to internal friction as they move. Most practical fluids, such as water, air, and oil, fall into this category. Real fluids are essential in studying fluid mechanics in real-world applications.
Newtonian fluids have a constant viscosity that does not change with the rate of deformation or shear rate. The flow behavior of these fluids follows Newton’s law of viscosity, where the shear stress is directly proportional to the shear rate. Examples of Newtonian fluids include water, air, and thin motor oil. These fluids are commonly encountered in daily life and industrial processes.
Non-Newtonian fluids have a viscosity that changes with the rate of deformation or shear rate. These fluids do not follow Newton’s law of viscosity and can exhibit various flow behaviors, such as shear thinning, shear thickening, or viscoelasticity. Examples of non-Newtonian fluids include ketchup, toothpaste, and cornstarch mixed with water. These fluids are often encountered in household and industrial applications where complex flow behavior is observed.
Incompressible fluids have a constant density, regardless of changes in pressure. Most liquids are considered incompressible because their density does not significantly change under normal conditions. Water is a common example of an incompressible fluid. Incompressible fluids are crucial in hydraulic systems and fluid mechanics calculations where volume changes are negligible.
Compressible fluids have a density that changes significantly with pressure. Gases are typically compressible because their volume and density vary with pressure and temperature changes. Air is a common example of a compressible fluid. Understanding compressible fluids is essential in aerodynamics, gas dynamics, and high-speed fluid flow applications.
Density:
Density (ρ) is the mass per unit volume of a fluid. It influences buoyancy, pressure, and fluid dynamics. The formula for density is ρ=mv, where mmm is the mass and v is the volume. The typical unit is kilograms per cubic meter (kg/m³).
Viscosity:
Viscosity (μ) measures a fluid’s resistance to deformation or flow. It indicates how “thick” or “thin” a fluid is. High viscosity fluids, like honey, resist flow, while low viscosity fluids, like water, flow easily. Viscosity is crucial in determining the flow behavior of fluids in various applications.
Pressure:
Pressure (P) is the force exerted by a fluid per unit area. It is a scalar quantity essential in understanding fluid statics and dynamics. The standard unit of pressure is the Pascal (Pa), defined as one Newton per square meter (N/m²). Fluid pressure can be measured using instruments like manometers and barometers.
Temperature:
Temperature (T) affects the physical properties of fluids, such as density and viscosity. For example, heating a fluid generally decreases its viscosity and density. Temperature is a fundamental thermodynamic property that influences the state and behavior of fluids.
Compressibility:
Compressibility measures how much a fluid’s volume decreases under pressure. It is significant for gases, which are highly compressible, and less so for liquids, which are nearly incompressible. Compressibility affects fluid dynamics, especially at high velocities and pressures.
Surface Tension:
Surface tension (γ) is the elastic tendency of a fluid surface to minimize its surface area. It causes liquids to form droplets and affects phenomena like capillary action. Surface tension results from cohesive forces between fluid molecules at the surface.
Buoyancy:
Buoyancy is the upward force exerted by a fluid on an immersed object, making it float or sink. Archimedes’ principle states that the buoyant force is equal to the weight of the displaced fluid. Buoyancy depends on the density difference between the fluid and the object.
Capillarity:
Capillarity is the ability of a fluid to flow in narrow spaces without external forces, often against gravity. This phenomenon is driven by cohesive and adhesive forces and is observed in processes like the movement of water in plant roots and soil.
Flow Rate:
Flow rate is the volume of fluid passing through a cross-section per unit time. It is a critical parameter in fluid dynamics and is measured in units like liters per second (L/s) or cubic meters per second (m³/s). Flow rate determines the efficiency and behavior of fluid systems.
Specific Gravity:
Specific gravity is the ratio of the density of a fluid to the density of a reference substance (usually water for liquids and air for gases). It is a dimensionless quantity used to compare the density of different fluids. Specific gravity is crucial in applications like fluid separation and material selection.
To help understand the properties of fluids, here are some practice problems. Each problem includes a detailed solution
Question: A cylindrical container with a radius of 5 cm and a height of 10 cm is filled with a liquid. The mass of the liquid is 2 kg. Calculate the density of the liquid.
Solution:
Calculate the volume of the cylinder: V=πr²h V = π(0.05)² (0.1)≈7.85×10⁻⁴m³
Calculate the density: ρ = m/v => ρ= 2kg/ 7.85×10⁻⁴m³ ≈ 2548n kg/m³
Answer: The density of the liquid is 2548 kg/m³.
Question: What is the pressure at a depth of 5 meters below the surface of a lake? (Assume the density of water is 1000 kg/m³ and acceleration due to gravity is 9.81 m/s²).
Solution:
Calculate the pressure: P=ρgh P=1000 kg/m³×9.81m/s² ×5m => P=49050Pa
Answer: The pressure at a depth of 5 meters is 49050 Pa (Pascals).
Liquids have a definite volume but no fixed shape, while gases have neither a definite volume nor a fixed shape.
Buoyancy is the upward force exerted by a fluid on an immersed object, allowing it to float or sink.
Fluid pressure is the force exerted per unit area by a fluid on a surface.
Pascal’s Principle states that a change in pressure applied to an enclosed fluid is transmitted equally throughout the fluid.
Bernoulli’s Principle states that an increase in fluid speed results in a decrease in pressure or potential energy.
The continuity equation states that the mass flow rate of a fluid remains constant from one cross-section of a pipe to another.
Laminar flow is smooth and orderly, while turbulent flow is chaotic and irregular.
Temperature can change the viscosity and density of a fluid, affecting its flow characteristics.
Surface tension is the cohesive force at the surface of a liquid, affecting phenomena like capillary action.
Archimedes’ Principle states that the buoyant force on an object is equal to the weight of the fluid displaced by the object.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary factor that determines the pressure in a fluid?
The density of the fluid
The volume of the fluid
The shape of the container
The temperature of the fluid
Which principle states that the pressure applied to a confined fluid is transmitted equally in all directions?
Archimedes' Principle
Bernoulli's Principle
Pascal's Principle
Newton's Law of Viscosity
What is the SI unit of pressure?
Pascal (Pa)
Newton (N)
Joule (J)
Watt (W)
What is the buoyant force on an object submerged in a fluid equal to?
The weight of the object
The volume of the object
The weight of the fluid displaced by the object
The density of the object
What does Bernoulli's Equation relate to?
Temperature and pressure
Density and velocity of a fluid
Pressure and velocity in a fluid flow
Mass and volume of a fluid
In a viscous fluid, what happens to the flow rate if the viscosity increases?
It increases
It decreases
It remains the same
It becomes unpredictable
What is the name of the phenomenon where a fluid's flow is smooth and orderly?
Turbulent flow
Laminar flow
Compressible flow
Incompressible flow
The continuity equation for incompressible fluids states that:
Density is constant
The product of density and velocity is constant
The product of velocity and cross-sectional area is constant
The sum of pressures is constant
What effect does increasing the temperature of a fluid typically have on its viscosity?
Viscosity increases
Viscosity decreases
Viscosity remains unchanged
Viscosity fluctuates
What is the term for the force exerted by a fluid against a surface?
Buoyant force
Viscous force
Pressure force
Gravitational force
Before you leave, take our quick quiz to enhance your learning!

