What is the SI unit of electric flux?
Coulomb
Newton
Volt
Volt-meter

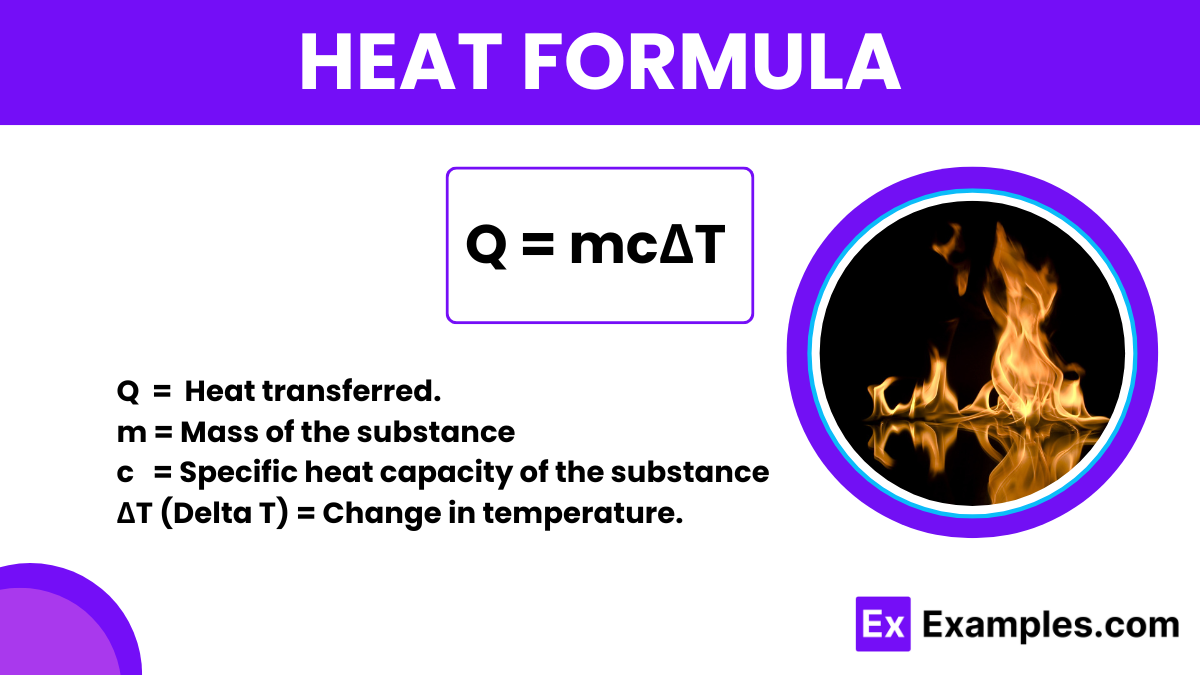
Heat refers to the transfer of thermal energy between physical systems, driven primarily by the temperature difference between them. In the context of physics, specifically thermodynamics, the formula to calculate the amount of heat transferred in a process involving temperature change in an object is given by:
This formula is pivotal as it connects the physical properties of materials with the thermal energy they exchange.
To derive this formula, consider a substance that absorbs or releases heat energy. The change in its internal energy due to temperature change is quantified by the specific heat capacity c, which is the amount of heat required to change the temperature of one kilogram of the substance by one degree Celsius. The total heat change (Q) is the product of the mass of the substance, its specific heat, and the change in temperature. This relationship is intuitive: a larger mass or a greater temperature change involves a greater heat transfer.
This formula was developed through the foundational works on thermodynamics, largely attributed to scientists such as Joseph Black, who discovered specific heat in the 18th century, and later James Prescott Joule, who demonstrated the mechanical equivalent of heat. These discoveries laid the groundwork for the quantitative analysis of heat transfer and the formulation of the first law of thermodynamics.
Question: How much heat is required to raise the temperature of 150 kg of water from 20°C to 45°C? Assume the specific heat capacity of water is 4.186 J/g°C4.186 J/g°C.
Solution: First, convert the mass of water to grams because the specific heat capacity is in J/g°C: 𝑚=150,000 g
Use the heat formula: 𝑄=𝑚 x 𝑐 x Δ𝑇
𝑄=150,000 g×4.186 J / g°C × (45°𝐶−20°𝐶)
Q=150,000 g×4.186 J / g°C × 25°C
𝑄=15,697,500 J
Therefore, 15,697,500 Joules of heat are required.
Question: A 500 g copper rod at 150°C needs to be cooled to 25°C. The specific heat capacity of copper is 0.385 J/g°C. How much heat must be released?
Solution: Calculate the change in temperature:
Δ𝑇=25°𝐶−150°𝐶=−125°𝐶
Now apply the heat formula:
𝑄=𝑚𝑐Δ𝑇
𝑄=500 g × 0.385 J/g°C × (−125°𝐶)
𝑄=−24,062.5 J
The negative sign indicates heat release. Therefore, the copper rod releases 24,062.5 Joules of heat.
Question: Calculate the heat required to increase the temperature of 200 g of aluminum from 22°C to 55°C. The specific heat capacity of aluminum is 0.897 J/g°C.
Solution: Calculate the temperature increase:
Δ𝑇=55°𝐶−22°𝐶=33°𝐶
Now, apply the formula:
𝑄=𝑚𝑐Δ𝑇
𝑄=200 g×0.897 J/g°C×33°𝐶
𝑄=5,921.4 J
5,921.4 Joules of heat is needed to warm the aluminum.
The formula 𝑄=𝑚𝐿 calculates heat required for phase changes, where m is mass and L is latent heat.
Yes, in thermodynamics, Q symbolizes the quantity of heat transferred in or out of a system.
The letter Q is used for heat in equations to represent the total energy transferred due to temperature differences.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the SI unit of electric flux?
Coulomb
Newton
Volt
Volt-meter
Which of the following units is equivalent to Newton-meter squared per coulomb?
Volt-meter
Joule-meter
Coulomb-meter
Tesla-meter
What is the dimensional formula of electric flux?
[MLT⁻²Q⁻¹]
[ML²T⁻²Q⁻¹]
[MLT⁻¹Q⁻²]
[ML²T⁻²Q]
If the electric field is measured in volts per meter, what is the unit of electric flux through a surface?
Volt
Coulomb
Newton
Volt-meter
How is electric flux related to the electric field and area vector?
It is the product of electric field and area vector
It is the sum of electric field and area vector
It is the difference between electric field and area vector
It is the ratio of electric field and area vector
What is the unit of electric flux density?
Volt per meter
Newton per coulomb
Coulomb per meter squared
Joule per meter squared
In the CGS system, what is the unit of electric flux?
Statvolt
Gauss
Dyne-centimeter
Statvolt-centimeter
What quantity does the unit Newton-meter squared per coulomb represent?
Electric potential
Electric flux
Electric charge
Electric current
Which law directly involves electric flux?
Ohm's Law
Gauss's Law
Faraday's Law
Faraday's Law
If the electric field is uniform and the surface is flat, how is electric flux calculated?
E/A
E + A
E - A
E·A
Before you leave, take our quick quiz to enhance your learning!

