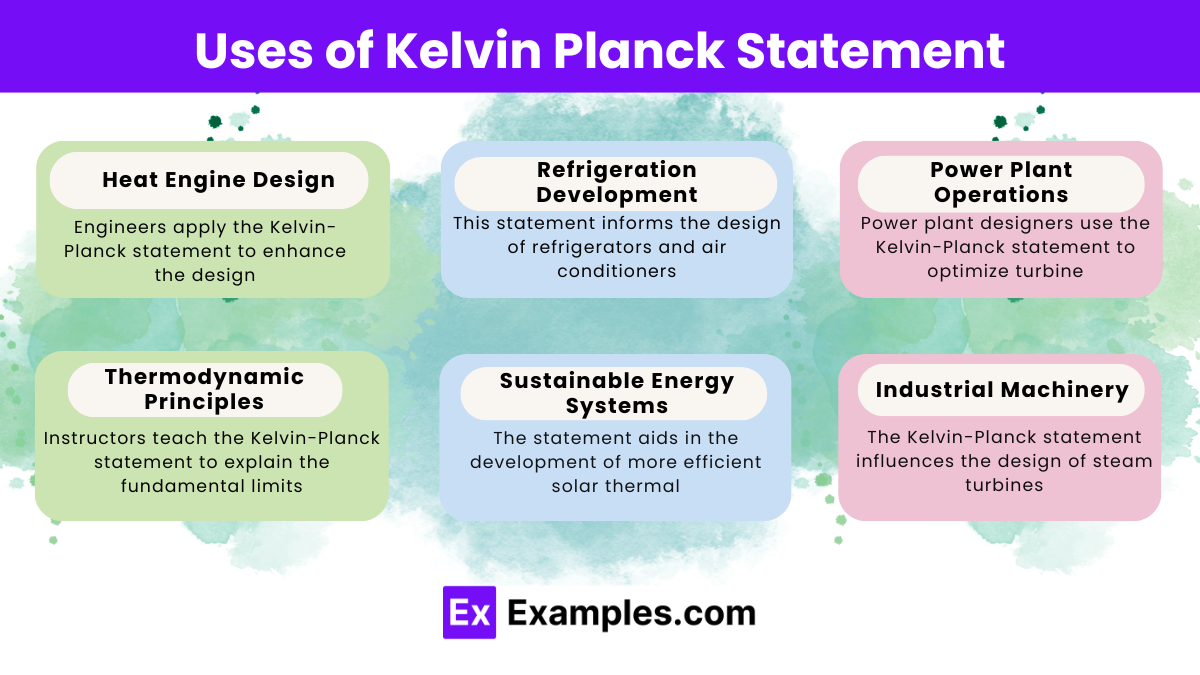
What is the Kelvin-Planck statement of the second law of thermodynamics?
No process is possible in which the sole result is the transfer of energy as heat from a cooler to a hotter body
No process is possible whose sole result is the absorption of heat from a reservoir and the conversion of this heat into work
Energy cannot be created or destroyed, only transformed
Entropy of an isolated system always increases