What does the Law of Conservation of Energy state?
Energy cannot be created or destroyed, only transformed
Energy can be created from nothing
Energy can be destroyed and recreated
Energy is always conserved in isolated systems only

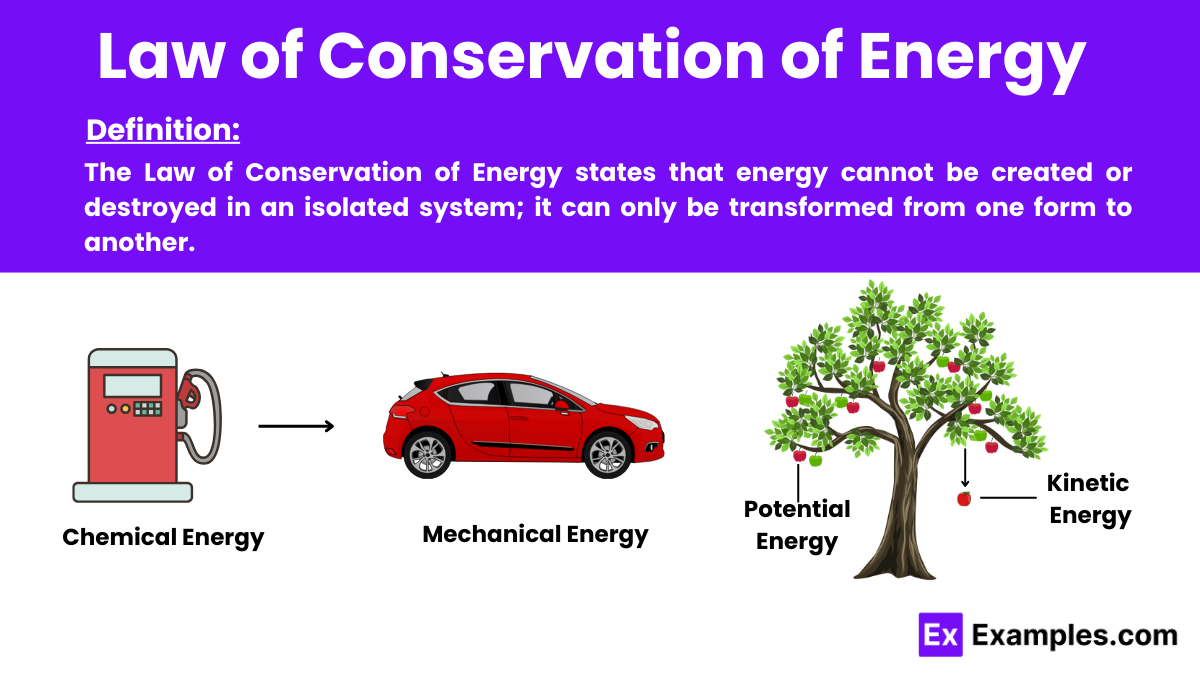
In physics, the Law of Conservation of Energy states that energy cannot be created or destroyed in an isolated system; it can only be transformed from one form to another. This fundamental principle is one of the key laws of physics and underscores the constancy of energy in all physical processes, whether it involves mechanical, thermal, chemical, or electrical energy transformations.
The Law of Conservation of Energy manifests in several forms, each relevant to different physical contexts:
In mechanical systems, energy shifts between kinetic energy (movement energy) and potential energy (stored energy due to position). For example, a swinging pendulum exchanges its height (potential energy) for speed (kinetic energy) and back.
This form deals with heat energy. When energy transforms into heat, as with friction, it remains within the system, complying with conservation principles, such as in insulated systems where heat does not escape.
In chemical reactions, the energy stored in bonds of reactants transforms into products or releases as heat, yet the total energy remains constant. This principle is crucial for understanding reactions in batteries or metabolic processes in biology.
In electrical circuits, energy converts from electrical potential to other forms like light or heat without loss of total system energy, important for power management and efficiency in electrical systems.
The formula for the Law of Conservation of Energy is straightforward:
This equation states that the total energy 𝐸ₜₒₜₐₗ of an isolated system remains constant. Meaning the initial energy 𝐸ᵢₙᵢₜᵢₐₗ before any processes occur equals the final energy 𝐸բᵢₙₐₗ after those processes. This encapsulates all forms of energy, including kinetic, potential, thermal, and others, ensuring their sum remains unchanged regardless of the transformations that occur within the system
The derivation of the Law of Conservation of Energy formula is grounded in the fundamental principle that energy cannot be created or destroyed, only transformed from one form to another. Here’s a simplified explanation of how this principle leads to the conservation of energy equation:
Energy can exist in various forms such as kinetic, potential, thermal, electrical, and chemical. Each form can be quantitatively described by specific equations. Like kinetic energy 𝐾𝐸=1/2𝑚𝑣² and potential energy 𝑃𝐸=𝑚𝑔ℎ, where:
Consider a closed system where energy transformations occur without any external energy input or loss to the surroundings. For instance, a pendulum swinging back and forth converts its energy from kinetic to potential and back without losing energy to the environment.
In any process within a closed system, the total energy at any time can be represented by the sum of all forms of energy present. For the swinging pendulum:
By setting up an equation that balances the total energies at different points in time or different states of the system. You acknowledge that the sum of energies remains constant. Mathematically, this is expressed as: Total Energy at Start=Total Energy at End 𝐸ᵢₙᵢₜᵢₐₗ=𝐸բᵢₙₐₗ
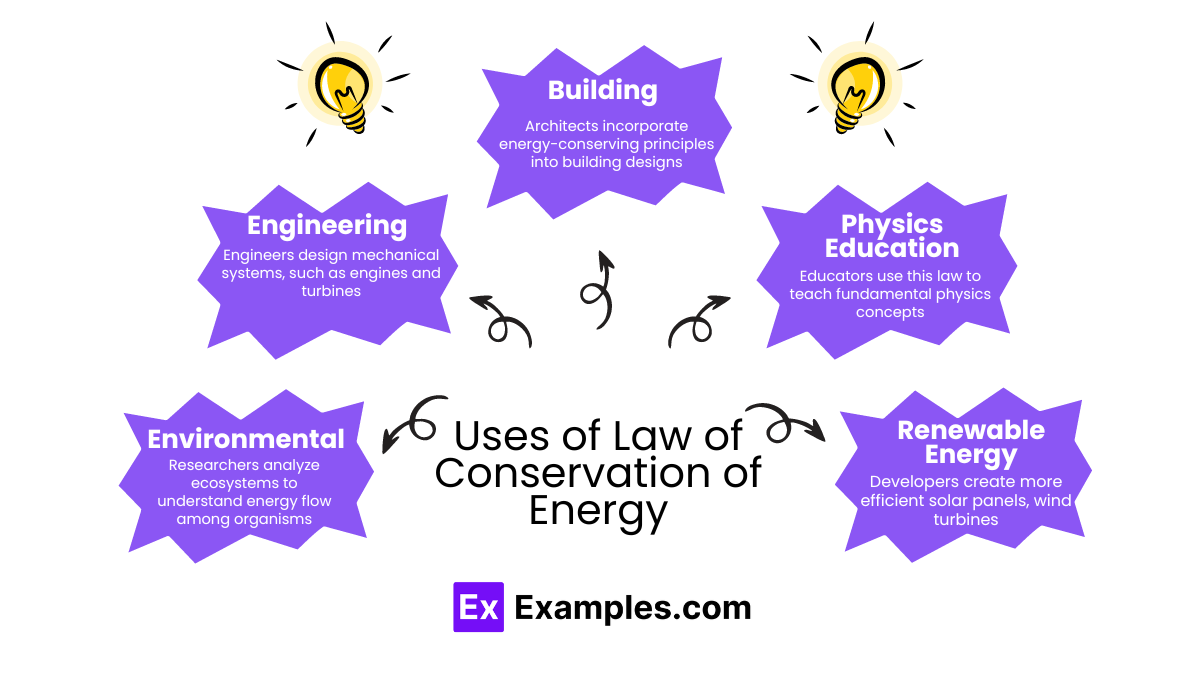
The Law of Conservation of Energy has wide-ranging applications across various fields, demonstrating its fundamental role in both theoretical and practical aspects:
Here are practical examples demonstrating the Law of Conservation of Energy in everyday and scientific contexts:
According to the Law of Conservation of Energy, energy simply transforms from one form to another, maintaining constant total energy in a closed system.
In black holes, energy is not destroyed; rather, it becomes trapped indefinitely, effectively removing it from the observable universe but still conserved.
The law asserts that energy remains constant. It transforms between different forms, and cannot disappear or emerge from nothing in an isolated system.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does the Law of Conservation of Energy state?
Energy cannot be created or destroyed, only transformed
Energy can be created from nothing
Energy can be destroyed and recreated
Energy is always conserved in isolated systems only
Which of the following is an example of energy transformation?
A rock sitting on a hill
A car moving on a highway
A book lying on a table
A piece of paper burning
In an isolated system, how does the total energy change?
It can increase or decrease
It remains constant
It fluctuates randomly
It doubles every second
How does the Law of Conservation of Energy apply to a pendulum in motion?
Potential energy is converted into kinetic energy and vice versa
Energy is lost to friction and air resistance
Energy is gained from the surroundings
Energy remains constant only if the pendulum is stationary
Which scenario demonstrates the Law of Conservation of Energy?
A ball thrown up in the air gains more energy as it rises
An engine converts fuel into mechanical work
An object gains energy without any external input
A car’s speed decreases without any loss of energy
What happens to the energy in a closed system during a collision?
Energy is created
Energy is destroyed
Energy is transformed but conserved
Energy changes unpredictably
How does the Law of Conservation of Energy apply to a roller coaster ride?
The total mechanical energy changes as the coaster moves
Potential energy increases as the coaster descends
Kinetic energy increases as the coaster ascends
Total energy remains constant, with transformations between potential and kinetic energy
What is the primary principle behind energy conservation in a thermodynamic system?
Energy can only be converted into heat
Energy is conserved and transformed into different types
Energy is destroyed when heat is lost
Energy is created to match work done
Which statement about energy in a chemical reaction is true?
Energy is neither created nor destroyed but transformed
Energy is created from reactants
Energy is only destroyed
Energy is constant only if the reaction is in equilibrium
In a frictionless environment, how does the energy of a moving object change?
It decreases due to energy loss
It increases with external force
It remains constant as kinetic energy
It converts into thermal energy
Before you leave, take our quick quiz to enhance your learning!

