What does the Law of Conservation of Mass state?
Mass can be created and destroyed
Mass cannot be created or destroyed
Energy can be converted into mass
Mass can be converted into energy

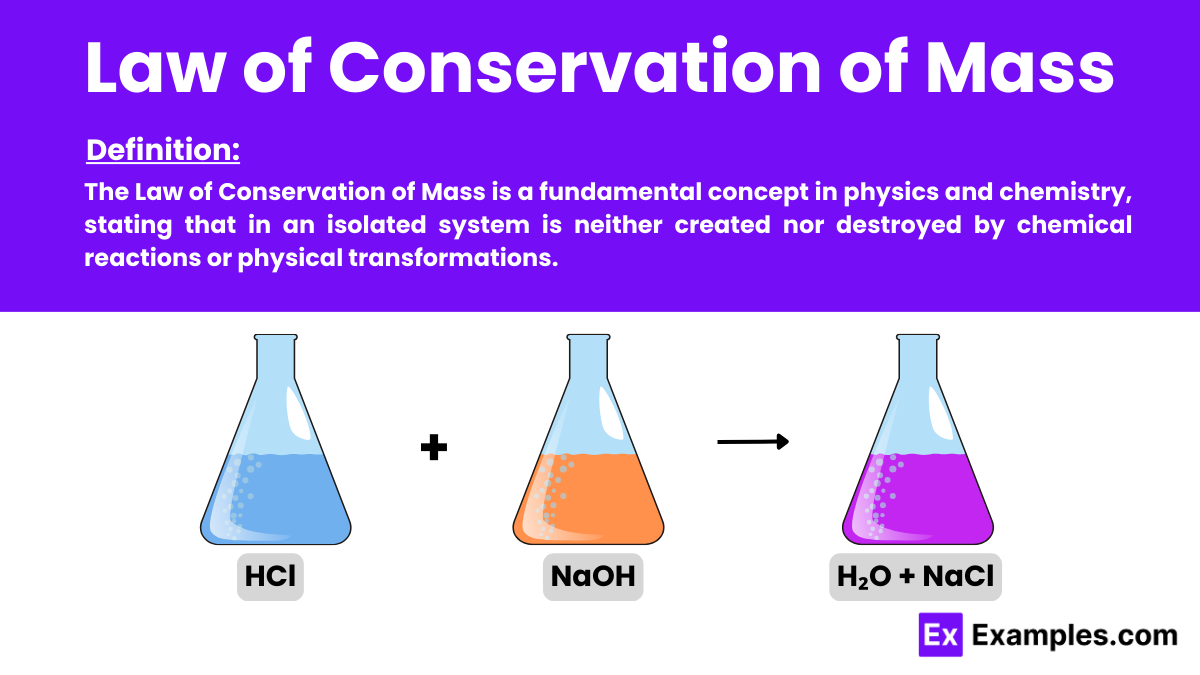
The Law of Conservation of Mass in physics asserts that mass is neither created nor destroyed in a closed system. During any physical or chemical process, the total mass of the system remains constant. This fundamental principle is key to understanding chemical reactions and physical transformations.
In physics, the principle of conservation of mass is fundamental and manifests primarily in two contexts: closed systems and isolated systems.
In closed systems, no matter can enter or leave the system, conserving mass. Observers often note this during chemical reactions within sealed containers, where the total mass of reactants matches that of the products despite transformations.
Isolated systems extend the principle further by preventing not only mass from moving in and out but also stopping energy exchanges with the environment. This type of system, more theoretical and idealized, serves as an important model in thermodynamics and statistical mechanics.
In physics, the formula for the Law of Conservation of Mass states:
This equation implies that the total mass 𝑚 of a system remains constant over time. Regardless of the processes occurring within the system. The mass before any reaction or physical change 𝑚ᵢₙᵢₜᵢₐₗ is equal to the mass after the change 𝑚բᵢₙₐₗ.
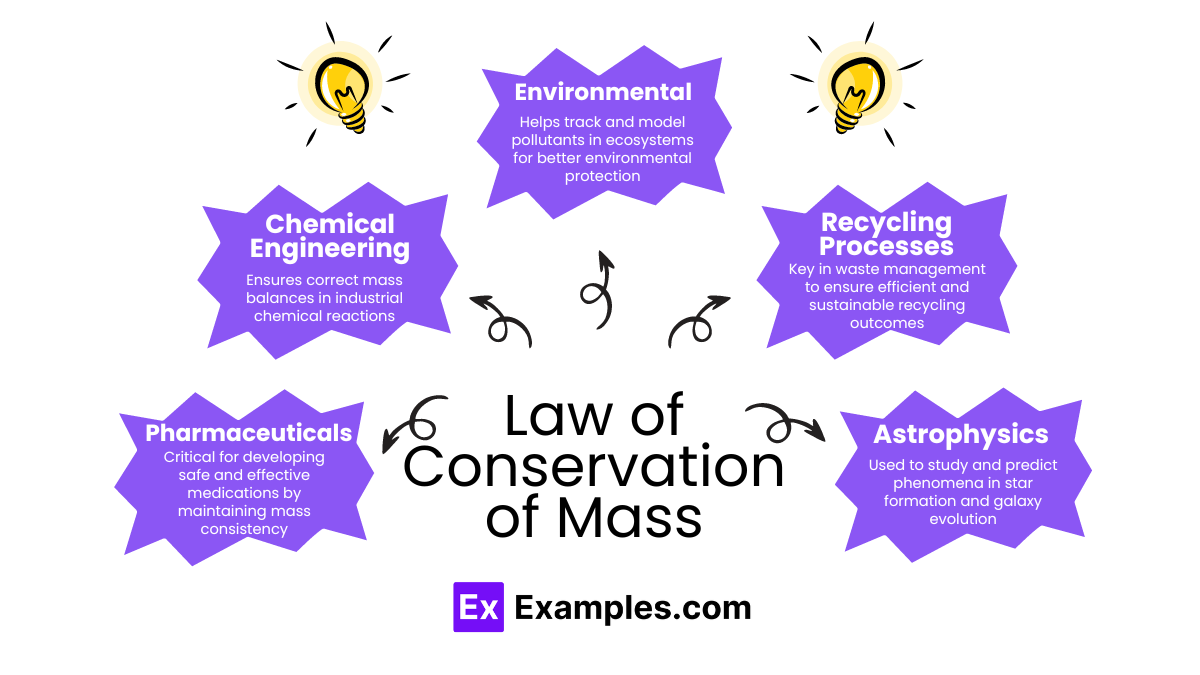
The Law of Conservation of Mass is vital across various fields, providing essential insights and efficiencies:
The Law of Conservation of Mass is evident in a variety of everyday and scientific scenarios:
It states that in any physical or chemical change, the total amount of mass stays the same; nothing gets lost or created.
The Law of Conservation of Mass was formulated by Antoine Lavoisier, a French chemist, in the late 18th century. He is often called the father of modern chemistry.
The father of conservation of mass is Antoine Lavoisier. He formulated the principle that in a closed system, mass is neither created nor destroyed.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does the Law of Conservation of Mass state?
Mass can be created and destroyed
Mass cannot be created or destroyed
Energy can be converted into mass
Mass can be converted into energy
Who is credited with formulating the Law of Conservation of Mass?
Isaac Newton
Albert Einstein
Antoine Lavoisier
Marie
In a chemical reaction, if the mass of reactants is 50 grams, what will be the mass of the products?
25 grams
50 grams
75 grams
100 grams
What must remain constant in a closed system according to the Law of Conservation of Mass?
Volume
Mass
Energy
Temperature
Which type of reactions does the Law of Conservation of Mass apply to?
Chemical reactions
Physical reactions
Nuclear react
All of the above
How is the Law of Conservation of Mass demonstrated in a chemical equation?
By showing equal energy on both sides
By showing equal mass of reactants and products
By balancing electrons on both sides
By showing equal volume on both sides
Which of the following is an example of the Law of Conservation of Mass?
Melting of ice
Dissolving salt in water
Combustion of wood
All of the above
What happens to the total mass of a system during a chemical reaction in a closed container?
It increases
It decreases
It remains the same
It fluctuates
If 10 grams of hydrogen reacts with 80 grams of oxygen, what is the total mass of water produced?
70 grams
80 grams
90 grams
100 grams
Why is the Law of Conservation of Mass important in chemical engineering?
To design energy-efficient processes
To ensure the mass balance in reactions
To calculate reaction rates
To determine product purity
Before you leave, take our quick quiz to enhance your learning!

