What does the Maxwell-Boltzmann distribution describe?
Distribution of particle speeds in a gas
Distribution of electric charges in a conductor
Distribution of magnetic fields in a material
Distribution of photons in a light beam

Maxwell-Boltzmann Distribution Law is a fundamental principle in physics that describes the distribution of particle speeds in a gas. This law of physics applies to ideal gases and states that the fraction of particles moving at a specific speed depends on the temperature and the particle’s mass. The distribution curve shows that most particles move at an average speed, with fewer particles moving at much higher or lower speeds. This law is essential in statistical mechanics. Providing insight into the kinetic behavior of gas molecules.
The formula for the Maxwell-Boltzmann Distribution Law describes the distribution of speeds of particles in an ideal gas. It is expressed as:
where:
The formula provides the probability of finding a particle with a particular speed at a given temperature. It shows that the speed distribution is skewed, with most particles having a moderate speed, and fewer particles having very low or very high speeds.
The Maxwell-Boltzmann Distribution Law provides the statistical distribution of speeds for particles in an ideal gas. Here’s a simplified derivation:
Energy of a Particle: Consider a single gas particle with kinetic energy 𝐸=1/2𝑚𝑣². Where 𝑚 is the particle’s mass and v is its speed.
Distribution of Energies: The probability of finding a particle with a specific energy follows the Boltzmann distribution, which is 𝑃(𝐸)∝𝑒−𝐸𝑘𝑇. Here, 𝑘 is the Boltzmann constant, and 𝑇 is the absolute temperature.
Probability Distribution: Since 𝐸=1/2𝑚𝑣², the speed distribution becomes: 𝑃(𝑣)∝𝑣²𝑒^−𝑚𝑣²/2𝑘𝑇. The 𝑣² term arises because we consider the number of possible states for each energy level.
Normalization: To find the exact distribution, the function must be normalized to integrate to 1 over all possible speeds. This gives the full Maxwell-Boltzmann distribution: 𝑓(𝑣)=4𝜋(𝑚/2𝜋𝑘𝑇)^3/2𝑣²𝑒^−𝑚𝑣²/2𝑘𝑇
This formula describes the probability distribution of speeds for gas particles, showing that most particles have a moderate speed, with fewer particles having very high or very low speeds.
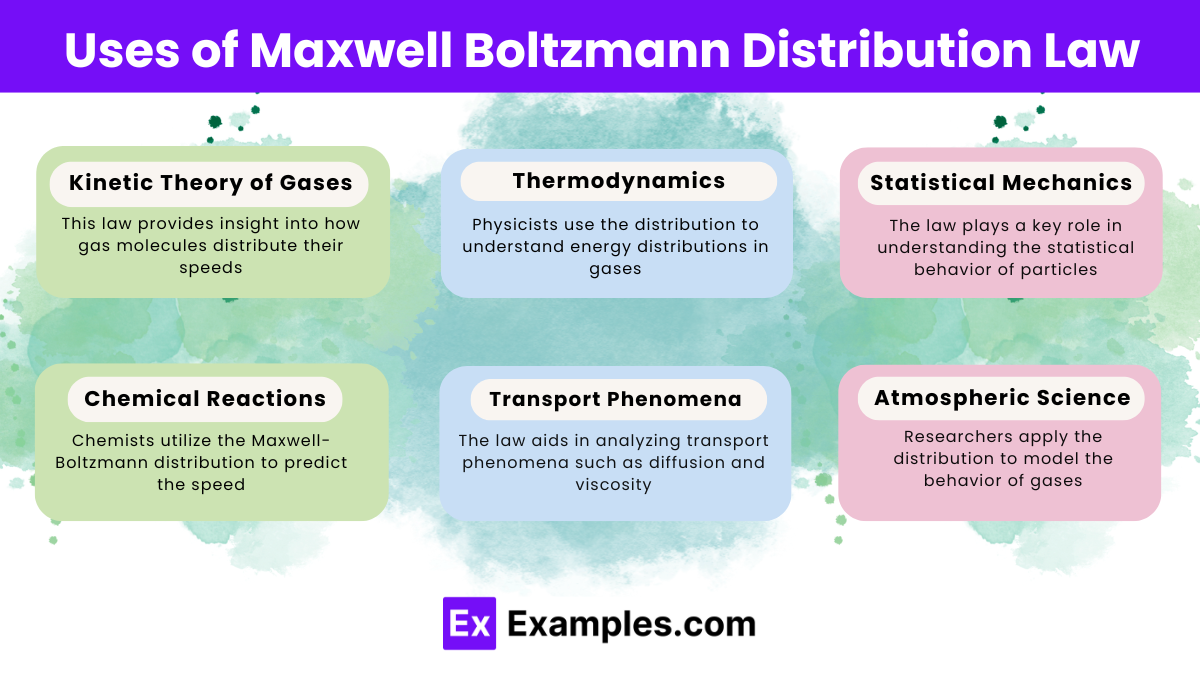
Temperature and particle mass significantly affect the distribution. Increased temperature shifts speeds higher, and lighter particles reach higher speeds than heavier ones.
The Boltzmann distribution is a probability density function, so it has units of 1/speed for the Maxwell-Boltzmann distribution.
Higher temperatures broaden the distribution, increasing the average particle speed, while lower temperatures narrow it, reducing particle speed.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does the Maxwell-Boltzmann distribution describe?
Distribution of particle speeds in a gas
Distribution of electric charges in a conductor
Distribution of magnetic fields in a material
Distribution of photons in a light beam
Which of the following is a key assumption of the Maxwell-Boltzmann distribution?
All particles have the same speed
Particles move in random directions
Particles do not collide
Particles have the same mass
In the context of the Maxwell-Boltzmann distribution, what does the term "most probable speed" refer to?
The average speed of particles
The speed at which the largest number of particles move
The maximum speed of particles
The minimum speed of particles
How does temperature affect the Maxwell-Boltzmann distribution of particle speeds?
Higher temperature shifts the distribution to higher speeds
Higher temperature shifts the distribution to lower speeds
Temperature has no effect
Higher temperature decreases the number of particles
What does the area under the Maxwell-Boltzmann distribution curve represent?
Total energy of the system
Total number of particles
Total pressure of the gas
Total volume of the gas
Which of the following is true about the shape of the Maxwell-Boltzmann distribution curve at very high temperatures?
It becomes narrower
It becomes broader
It becomes flat
It becomes more peaked
What does the Maxwell-Boltzmann distribution law imply about the kinetic energy of gas particles?
All particles have the same kinetic energy
Kinetic energy is uniformly distributed among particles
Kinetic energy varies and follows a specific distribution
Kinetic energy is inversely proportional to temperature
Which factor is directly proportional to the average kinetic energy of gas particles in the Maxwell-Boltzmann distribution?
Volume of the gas
Pressure of the gas
Temperature of the gas
Number of particles
How does the most probable speed compare to the average speed in a Maxwell-Boltzmann distribution?
Most probable speed is higher
Most probable speed is lower
They are equal
Relationship varies with temperature
What effect does increasing the mass of gas particles have on the Maxwell-Boltzmann distribution?
Shifts the distribution to higher speeds
Shifts the distribution to lower speeds
Broadens the distribution
Narrows the distribution
Before you leave, take our quick quiz to enhance your learning!

