What is Michael Faraday best known for in the field of science?
Discovery of the electron
Development of the theory of relativity
Contributions to electromagnetism and electrochemistry
Discovery of radioactivity
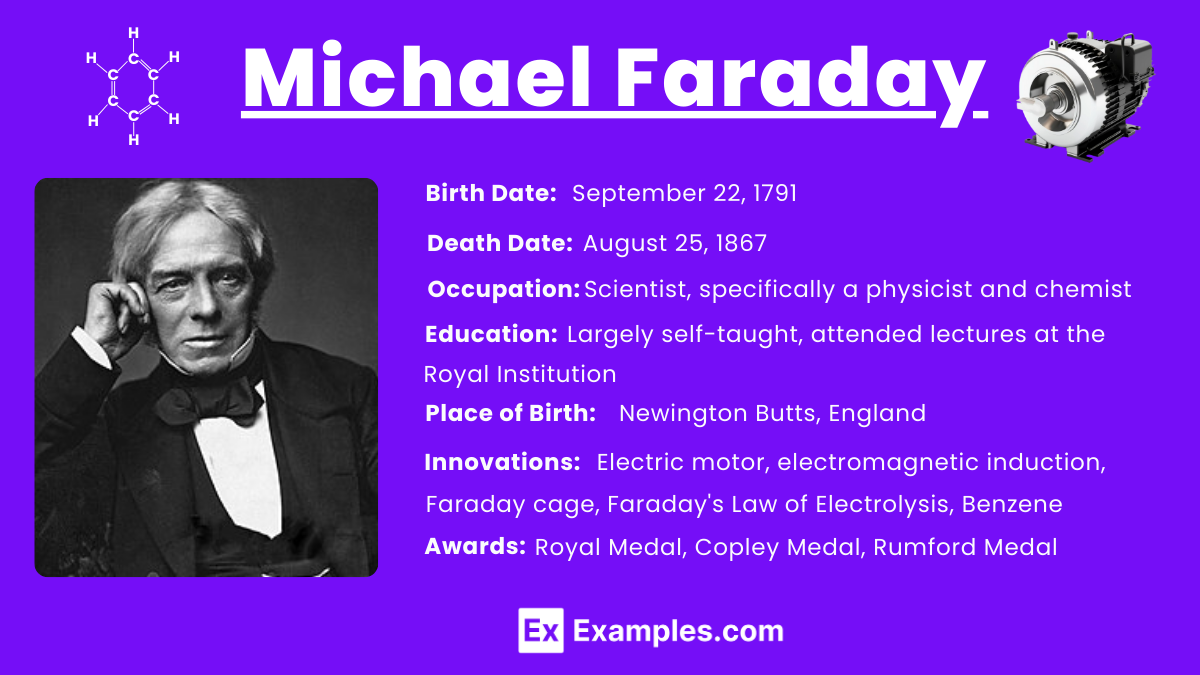
Michael Faraday was born in 1791 in Newington Butts, which is now part of South London. He grew up in a poor family and received only basic education in reading, writing, and arithmetic at a local church Sunday school. From an early age, Faraday demonstrated a keen interest in learning, particularly in the scientific fields, despite his limited access to formal education.
At the age of 14, Faraday began an apprenticeship with a local bookbinder and bookseller. Over the course of seven years, he used this opportunity to educate himself. He read many books, focusing on topics in chemistry, physics, and electricity. This self-directed study laid the groundwork for his future scientific achievements.
Faraday’s formal scientific education began when he attended lectures by the eminent chemist Sir Humphry Davy at the Royal Institution of Great Britain. In 1812, after sending Davy a 300-page book of notes he had taken during these lectures, Faraday was appointed as a chemical assistant at the Royal Institution. Here, he immersed himself in scientific research, experimenting and learning directly from practical work and further lectures. This hands-on experience in the lab was crucial in developing his understanding and skills in physical and chemical phenomena.
In 1831, Michael Faraday discovered electromagnetic induction, a principle that explains how electric currents generate magnetic fields and vice versa. He conducted experiments using coils and magnets, demonstrating that moving a magnet through a coil of wire produces an electric current. This discovery laid the foundation for the development of electric generators and transformers.
Faraday formulated the laws of electrolysis around 1833, quantifying the relationship between the amount of electrical charge used in an electrolysis experiment and the substance liberated at the electrode. His laws are still used today to predict and understand the behavior of ions in solutions during electrolysis.
Faraday invented the Faraday cage in 1836. This enclosure, made from conducting materials, shields its contents from static electric fields and electromagnetic radiation. The Faraday cage concept proves essential in protecting electronic equipment from lightning strikes and other electrostatic discharges.
In 1845, Faraday discovered diamagnetism, an effect exhibited by certain materials that repel magnetic fields. His research in this area further deepened scientific understanding of magnetic properties in various materials.
Faraday also contributed to the field of chemistry by identifying the compound benzene in 1825. His isolation of this hydrocarbon was significant because it opened the door to the study of aromatic compounds, which are crucial in the synthesis of many plastics, resins, and pharmaceuticals.
In 1831, Michael Faraday made the groundbreaking discovery of electromagnetic induction, revealing that a changing magnetic field induces an electric current in a nearby circuit. This principle is now fundamental in the operation of electric generators and transformers.
Following his experiments with electromagnetic induction, Faraday went on to build the first electric motor in the early 1820s. He demonstrated that electrical energy could convert into mechanical energy, thereby paving the way for the development of numerous mechanical devices powered by electricity.
Faraday formulated and published his two laws of electrolysis in 1834, which quantify how electric charge relates to chemical change at the electrodes during electrolysis. These laws help predict the amounts of different substances released or absorbed during the process.
In 1836, Faraday introduced the Faraday cage, an enclosure used to block external static and non-static electric fields. It effectively shields its contents from electric charges and electromagnetic radiation, a principle exploited in various applications to protect sensitive electronic equipment.
In 1825, Faraday discovered benzene during an experiment with illuminating gas, identifying it as a new hydrocarbon. This discovery was crucial in the later development of organic chemistry, influencing the synthesis of numerous chemical compounds.
Faraday’s research extended to the study of magnetism, where he discovered the principles of diamagnetism and paramagnetism in 1845. He showed that materials respond differently to magnetic fields, some being attracted and others repelled.
He passed away on August 25, 1867, at his house in Hampton Court. He was 75 years old. His health had been declining for several years before his death. Despite offers of burial in Westminster Abbey, Faraday chose to be buried in a simple grave at Highgate Cemetery in London, reflecting his modest life. His contributions to science, especially in electricity and magnetism, continue to influence the field of physics to this day.
Michael Faraday faced numerous failures, particularly early in his career, persisting through many unsuccessful experiments before achieving success.
Michael Faraday is most renowned for inventing the electric motor, a cornerstone in the development of electrical engineering.
Michael Faraday struggled with a lack of formal education and health issues later in life, which affected his ability to conduct research.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is Michael Faraday best known for in the field of science?
Discovery of the electron
Development of the theory of relativity
Contributions to electromagnetism and electrochemistry
Discovery of radioactivity
Which law states that the induced electromotive force in any closed circuit is equal to the negative of the time rate of change of the magnetic flux through the circuit?
Faraday's First Law of Electrolysis
Faraday's Second Law of Electrolysis
Faraday's Law of Electromagnetic Induction
Coulomb's Law
In which year did Michael Faraday discover electromagnetic induction?
1800
1821
1831
1855
What device did Michael Faraday invent that demonstrated the principle of electromagnetic induction?
Battery
Dynamo
Voltmeter
Transformer
Which of the following laws is associated with the relationship between electric charge and matter during electrolysis?
Ohm's Law
Faraday's Laws of Electrolysis
Ampere's Law
Kirchhoff's Laws
Faraday's First Law of Electrolysis states that the amount of chemical change during electrolysis is proportional to what?
The voltage applied
The resistance of the electrolyte
The current passed through the electrolyte
The duration of the electrolysis process
Which of the following concepts did Michael Faraday introduce in his study of electromagnetism?
Electric potential
Magnetic flux
Electric field
Electric field
What is the name of the lines used to represent the direction and strength of a magnetic field, as introduced by Faraday?
Electric lines
Magnetic poles
Magnetic lines of force
Electric flux
Michael Faraday held a prominent position at which scientific institution?
University of Cambridge
Royal Institution of Great Britain
Massachusetts Institute of Technology
University of Oxford
What was the title of the series of lectures given by Faraday that aimed to explain complex scientific concepts to a general audience?
The Royal Lectures
The Faraday Lectures
The Christmas Lectures
The Newtonian Lectures
Before you leave, take our quick quiz to enhance your learning!

