Who proposed the model of the atom with electrons in discrete energy levels?
Albert Einstein
Niels Bohr
Isaac Newton
Ernest Rutherford
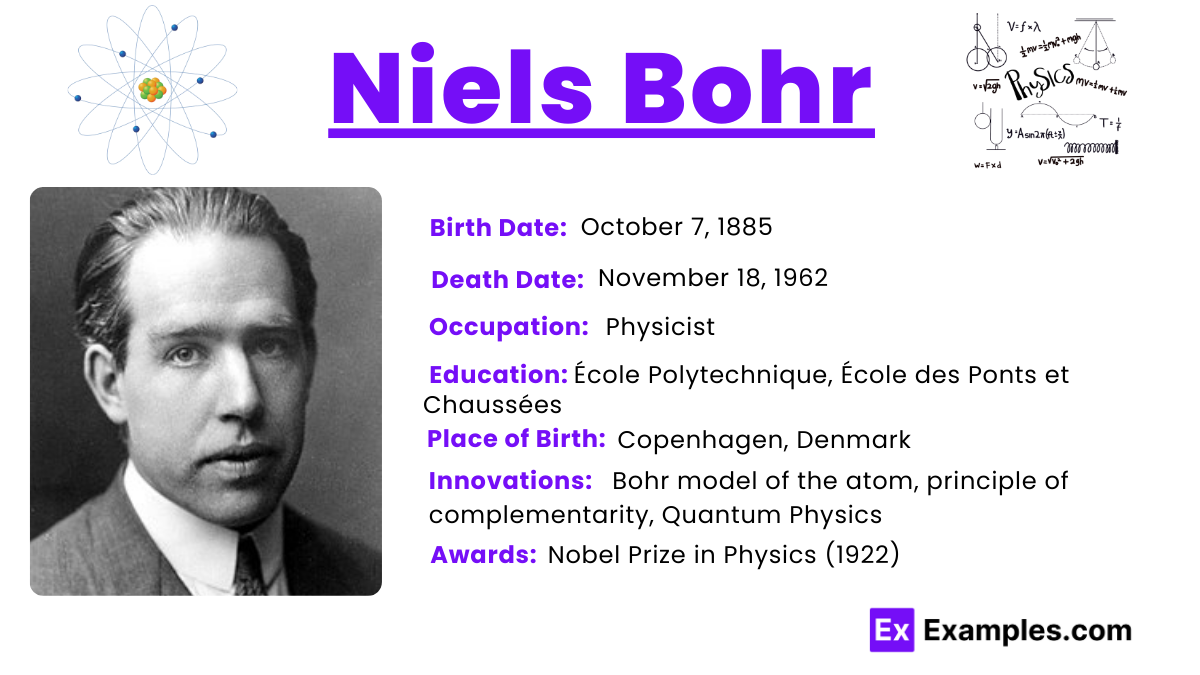
Niels Bohr was born into an educated and culturally rich family in Copenhagen, Denmark. His father, Christian Bohr, was a professor of physiology at the University of Copenhagen, and his mother, Ellen Adler Bohr, came from a prominent Jewish family well-known in the banking and parliamentary circles in Denmark. Growing up in an environment that valued learning and discussion, Bohr was deeply influenced by his academic surroundings. From a young age, he showed a keen interest in scientific inquiries, spurred by his father’s work and the intellectual atmosphere at home.
Niels Bohr began his formal education at Gammelholm Latin School in Copenhagen. During his time at Gammelholm, Bohr was known for his exceptional analytical skills and his profound interest in physics and mathematics. He graduated from Gammelholm in 1903, having displayed a strong aptitude in the sciences, which laid a solid foundation for his future studies.
After completing his schooling, Bohr enrolled at the University of Copenhagen in 1903. He initially studied philosophy and mathematics but soon shifted his focus entirely to physics. Bohr was greatly influenced by the works of his professors and the burgeoning field of quantum mechanics. His academic journey at the university was marked by intense study and research, leading to his master’s degree in Physics in 1909 followed by a doctorate in 1911. His doctoral thesis on the electron theory of metals introduced new ideas that later contributed to quantum mechanics, setting the stage for his future groundbreaking contributions to the field.
One of Niels Bohr’s first major contributions to physics was the Bohr model of the atom, introduced in 1913. This model suggested that electrons move in fixed orbits around the atom’s nucleus, and these orbits or energy levels are quantized, meaning electrons can only occupy certain allowed orbits. It effectively explained why atoms emitted light in fixed wavelengths, solving a fundamental problem of early quantum theory related to atomic structure.
Introduced in 1927, the principle of complementarity is a cornerstone of quantum mechanics. Bohr proposed that particles such as electrons possess both particle and wave properties. The characteristics can be observed separately under different conditions. This principle highlighted the dual nature of quantum phenomena, fundamentally challenging the traditional frameworks of physics and enhancing the understanding of particle-wave duality.
Bohr was instrumental in the development of quantum mechanics. His correspondence principle (1923) bridged the gap between the old quantum and the new quantum mechanics by stating that the behavior of systems. He described by quantum mechanics replicates classical physics in the limit of large quantum numbers. This principle was crucial in providing a smooth transition from classical to quantum systems.
In the 1930s, Niels Bohr’s work with John A. Wheeler led to the understanding of nuclear fission, the process by which a large nucleus splits into smaller nuclei while releasing energy and neutrons. This discovery was pivotal in the development of nuclear energy and weapons, laying the foundational principles for the atomic age.
While not an invention, the series of public debates between Bohr and Albert Einstein in the 1930s and 40s over the validity of quantum mechanics principles were highly significant. These debates primarily revolved around the uncertainty principle and quantum entanglement. Bohr defended the standard interpretation of quantum mechanics, advocating for its probabilistic nature against Einstein’s philosophical objections, which sought a more deterministic explanation of the universe.
In 1943, after Germany occupied Denmark, Bohr faced imminent danger due to his Jewish heritage. With the help of the Danish resistance and British agents, he fled Copenhagen. He escaped to Sweden and then traveled to the United Kingdom. Bohr worked on the British atomic bomb project, known as Tube Alloys, contributing his knowledge of nuclear fission.
Bohr moved to the United States where he became part of the Manhattan Project. The U.S.-led effort to develop atomic bombs. He worked at Los Alamos, New Mexico, where he interacted with prominent scientists like Robert Oppenheimer (scientific director of the project). Bohr provided theoretical insights that were crucial for the development of nuclear weapons.
Recognizing the devastating potential of nuclear weapons. Bohr advocated for the peaceful use of nuclear energy and the need for international cooperation to control atomic weapons. He met with influential leaders, including President Franklin D. Roosevelt and later Winston Churchill, to discuss the implications of nuclear weaponry. His efforts were initially met with skepticism and resistance. As the leaders were primarily focused on the war effort and the strategic advantage that nuclear weapons could provide.
Niels Bohr passed away on November 18, 1962, in Copenhagen, Denmark, after succumbing to heart failure. His death marked the end of a remarkable career in which he made foundational contributions to understanding atomic structure and quantum mechanics. Widely regarded as one of the most significant physicists of the 20th century. Bohr’s theories and principles continue to influence the scientific community. His legacy also includes his advocacy for the responsible use of atomic energy and his role in establishing CERN. The European Organization for Nuclear Research, reflecting his commitment to international scientific cooperation.
Niels Bohr pioneered significant contributions to modern physics through his groundbreaking work on atomic structure and quantum mechanics.
Niels Bohr’s famous quote is, “Prediction is very difficult, especially if it’s about the future.”
Yes, Niels Bohr met Albert Einstein, engaging in deep discussions and debates about quantum mechanics principles.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Who proposed the model of the atom with electrons in discrete energy levels?
Albert Einstein
Niels Bohr
Isaac Newton
Ernest Rutherford
In which year did Niels Bohr propose his model of the atom?
1911
1913
1920
1932
What is a key feature of Bohr's atomic model?
Electrons exist in fixed orbits
Electrons move in random paths
Atoms are indivisible
Electrons are embedded in a positive sphere
According to Bohr's model, how do electrons move between energy levels?
By spiraling inward
By slowly drifting
By jumping instantaneously
By colliding with the nucleus
What phenomenon did Bohr's model successfully explain?
Blackbody radiation
The photoelectric effect
Atomic spectra
Gravitational waves
Which concept from quantum theory is incorporated in Bohr's atomic model?
Wave-particle duality
Quantization of energy levels
Heisenberg's uncertainty principle
Schrödinger's wave equation
In Bohr's model, what happens when an electron transitions from a higher energy level to a lower one?
It absorbs energy
It emits energy
It loses mass
It changes its charge
What determines the frequency of the photon emitted during an electron transition in Bohr's model?
The electron's speed
The mass of the electron
The difference in energy levels
The charge of the nucleus
Bohr's model introduced which key idea about the stability of electron orbits?
Electrons are stable in any orbit
Electrons are stable only in certain discrete orbits
Electrons spiral into the nucleus
Electrons are unstable in all orbits
Which scientist's work on the atomic nucleus influenced Bohr's model?
Max Planck
Albert Einstein
Ernest Rutherford
J.J. Thomson
Before you leave, take our quick quiz to enhance your learning!

