What is Planck’s equation used to describe?
Motion of planets
Behavior of gases
Energy of photons
Speed of light

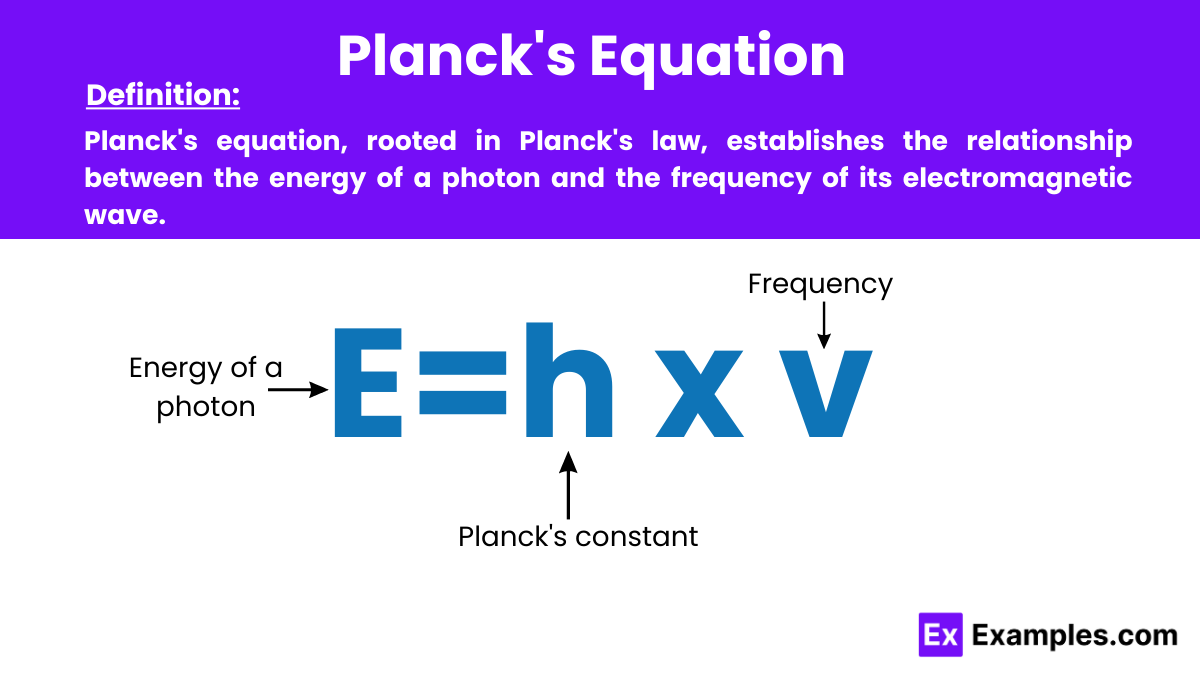
Planck’s Equation: In physics, Planck’s equation expresses the relationship between the energy of a photon and the frequency of its electromagnetic wave, foundational in quantum theory. It is formulated as E = hν, where E is the photon’s energy, h is Planck’s constant, and ν (nu) is the frequency. This equation is fundamental in the laws of physics, linking quantum mechanics and electromagnetic theory.
Planck’s constant is a fundamental constant in physics that defines the scale of quantum effects. Denoted by the symbol h, it quantifies the relationship between the energy of a photon and the frequency of its electromagnetic wave. Its value is approximately 6.626 × 10⁻³⁴ joule-seconds. This constant plays a critical role in quantum mechanics, particularly in Planck’s equation (E = hν), linking energy and frequency, and in the concept of quantization, where energy levels are discrete rather than continuous.
Planck’s equation formula is given by:
This formula shows that the energy of a photon is directly proportional to the frequency of its electromagnetic wave, and it forms the basis for understanding quantized energy in quantum mechanics.
Planck’s equation describes the relationship between a photon’s energy and the frequency of its electromagnetic wave, providing a quantized link fundamental to quantum mechanics.
The formula for Planck’s equation is E = hν, where E is energy, h is Planck’s constant, and ν (nu) represents the frequency of the wave.
Planck’s equation is crucial because it shows that energy is quantized, leading to the development of quantum mechanics and changing our understanding of atomic and subatomic behavior.
Planck’s constant is approximately 6.626 × 10⁻³⁴ joule-seconds, a universal constant used to relate the energy of photons to their frequency.
In the photoelectric effect, Planck’s equation relates the frequency of incoming light to the energy of emitted electrons, proving that light behaves as quantized photons.
Planck’s equation explains spectral lines by linking photon energy to specific frequencies, allowing scientists to identify elements based on their unique emission or absorption spectra.
Planck’s equation is widely used in quantum mechanics, providing insights into atomic energy levels, black-body radiation, and the quantization of electromagnetic radiation.
Planck’s equation helps explain the energy distribution in black-body radiation. Revealing how intensity varies with frequency and leading to the concept of quantized energy.
Technicians and engineers use Planck’s equation in technologies like LED lighting. X-ray generation, and photovoltaic cells to understand and optimize energy conversion and emission.
Planck’s equation challenged classical physics, introduced quantum theory, and reshaped our understanding of matter and energy on atomic and subatomic scales, revolutionizing scientific thought.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is Planck’s equation used to describe?
Motion of planets
Behavior of gases
Energy of photons
Speed of light
What is the formula for Planck’s equation?
E = mc²
E = hf
E = kT
E = mv²/2
What does the constant 'h' represent in Planck’s equation?
Boltzmann constant
Gravitational constant
Planck’s constant
Speed of light
What is the value of Planck’s constant?
3.00 x 10⁸ m/s
6.63 x 10⁻³⁴ Js
1.38 x 10⁻²³ J/K
9.81 m/s²
In the equation E = hf, what does 'f' stand for?
Force
Frequency
Faraday’s constant
Focal length
How does the energy of a photon change if its frequency is doubled?
It remains the same
It is halved
It doubles
It becomes zero
What is the unit of frequency 'f' in Planck’s equation?
Hertz (Hz)
Joules (J)
Meters (m)
Seconds (s)
Which phenomenon can be explained using Planck’s equation?
Photoelectric effect
Gravitational force
Thermal expansion
Electromagnetic induction
What happens to the energy of a photon if the wavelength decreases?
It remains the same
It decreases
It increases
It becomes zero
How is the frequency (f) related to the wavelength (λ) and speed of light (c)?
f = cλ
f = c/λ
f = λ/c
f = c²λ
Before you leave, take our quick quiz to enhance your learning!

