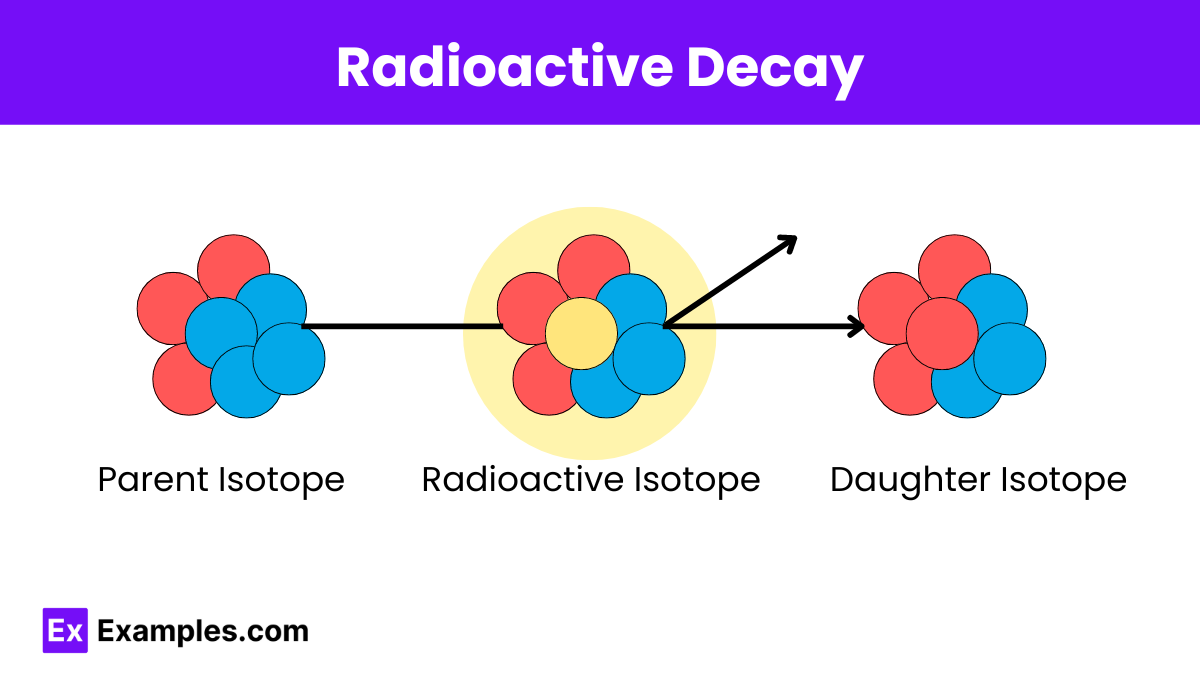
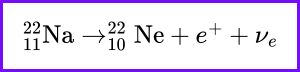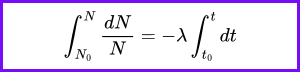What is the primary factor that determines the rate of radioactive decay of a substance?
The temperature of the substance
The concentration of the radioactive material
The half-life of the radioactive substance
The external pressure applied to the substance