What type of radiation is most penetrating and requires heavy shielding to stop?
Alpha radiation
Beta radiation
Gamma radiation
Neutron radiation

Radioactivity is the process by which unstable atomic nuclei lose energy by emitting radiation in the form of particles or electromagnetic waves. This phenomenon occurs naturally in certain elements, such as uranium, radium, francium, astatine, polonium, and radon, as their atoms decay over time to achieve a more stable state. Radioactive Decay Law describes the rate at which these unstable nuclei disintegrate.. This process is crucial in various applications, including medical imaging, cancer treatment, and as a power source in nuclear reactors and space missions.
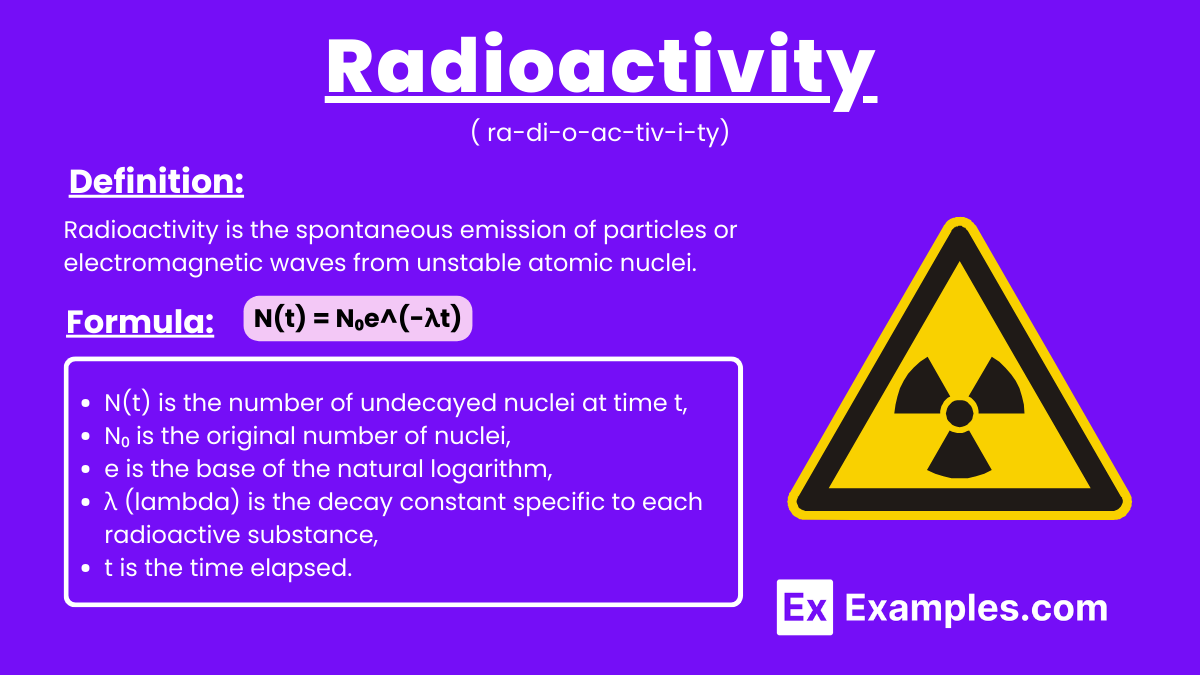
Radioactivity is the spontaneous emission of particles or electromagnetic waves from unstable atomic nuclei. This process results in the release of alpha particles, beta particles, or gamma rays, transforming the nuclei into more stable forms. Radioactivity occurs naturally in elements like uranium and can also be induced artificially, playing a vital role in medical treatments, energy production, and scientific research.
The formula that describes the rate of radioactive decay is given by:
N(t) is the number of undecayed nuclei at time t,
N₀ is the original number of nuclei,
e is the base of the natural logarithm,
λ (lambda) is the decay constant specific to each radioactive substance,
t is the time elapsed.
Here, units of radioactivity are listed below
Radioactivity can be classified into four main types based on the nature of the emitted particles or radiation:
Radioactivity has a wide range of applications across various fields due to its unique properties. Here are some of the key uses:
It occurs when atomic nuclei are unstable and undergo decay to achieve a more stable state.
Alpha, beta, and gamma radiation are common types emitted during radioactive decay.
Exposure can damage cells and DNA, potentially leading to cancer or other health issues.
Rocks, soil, and cosmic rays contribute to natural background radiation.
Geiger counters and dosimeters measure radiation levels in counts per minute or sieverts.
It’s the time for half of a radioactive substance to decay into a stable form.
Splitting of atomic nuclei, releasing energy (e.g., in nuclear reactors and bombs).
Radiation is energy emitted from a source, while radioactivity involves the emission of particles or waves from atomic nuclei.
It can affect ecosystems and organisms, depending on exposure levels.
Yes, through processes like neutron bombardment or particle accelerators to create radioactive isotopes.

Radioactivity is the process by which unstable atomic nuclei lose energy by emitting radiation in the form of particles or electromagnetic waves. This phenomenon occurs naturally in certain elements, such as uranium, radium, francium, astatine, polonium, and radon, as their atoms decay over time to achieve a more stable state. Radioactive Decay Law describes the rate at which these unstable nuclei disintegrate.. This process is crucial in various applications, including medical imaging, cancer treatment, and as a power source in nuclear reactors and space missions.
Radioactivity is the spontaneous emission of particles or electromagnetic waves from unstable atomic nuclei. This process results in the release of alpha particles, beta particles, or gamma rays, transforming the nuclei into more stable forms. Radioactivity occurs naturally in elements like uranium and can also be induced artificially, playing a vital role in medical treatments, energy production, and scientific research.
The formula that describes the rate of radioactive decay is given by:
N(t) = N₀e^(-λt)
N(t) is the number of undecayed nuclei at time t,
N₀ is the original number of nuclei,
e is the base of the natural logarithm,
λ (lambda) is the decay constant specific to each radioactive substance,
t is the time elapsed.
Here, units of radioactivity are listed below
SI Unit of Radioactivity = becquerel (Bq).
CGS unit of radioactivity : curie (Ci)
Uranium-238: Undergoes alpha decay, transforming into Thorium-234, and is used in nuclear reactors and geological dating.
Carbon-14: Undergoes beta decay, transforming into Nitrogen-14, and is essential for radiocarbon dating in archaeology and geology.
Radon-222: Produced from Radium-226 decay, it undergoes alpha decay into Polonium-218, and its levels are monitored due to health risks.
Plutonium-239: A man-made isotope undergoing alpha decay, crucial for nuclear power and weapons.
Thorium-232: Naturally decays into Lead-208 and has potential uses as a nuclear reactor fuel.
Potassium-40: Undergoes beta decay into Calcium-40, contributing to natural radioactivity in the human body and Earth’s crust.
Iodine-131: A product of nuclear fission, it undergoes beta decay and is used in medical treatments for thyroid conditions.
Cesium-137: Undergoes beta decay into Barium-137m, and is used in medical and industrial applications, such as cancer treatment and food irradiation.
Strontium-90: A byproduct of nuclear fission, it undergoes beta decay and is a concern in nuclear fallout due to its similarity to calcium.
Polonium-210: Undergoes alpha decay into Lead-206 and is highly toxic, used in industrial applications and as a poison.
Americium-241: A man-made isotope used in smoke detectors, it undergoes alpha decay.
Radium-226: Undergoes alpha decay into Radon-222, and was historically used in luminous paints and medical treatments.
Technetium-99m: Used in medical imaging, it undergoes gamma decay and has a short half-life, making it ideal for diagnostic purposes.
Tritium (Hydrogen-3): Undergoes beta decay and is used in self-luminous devices and nuclear fusion research.
Cobalt-60: Undergoes beta decay into Nickel-60, and is used in radiation therapy for cancer treatment and in industrial radiography.

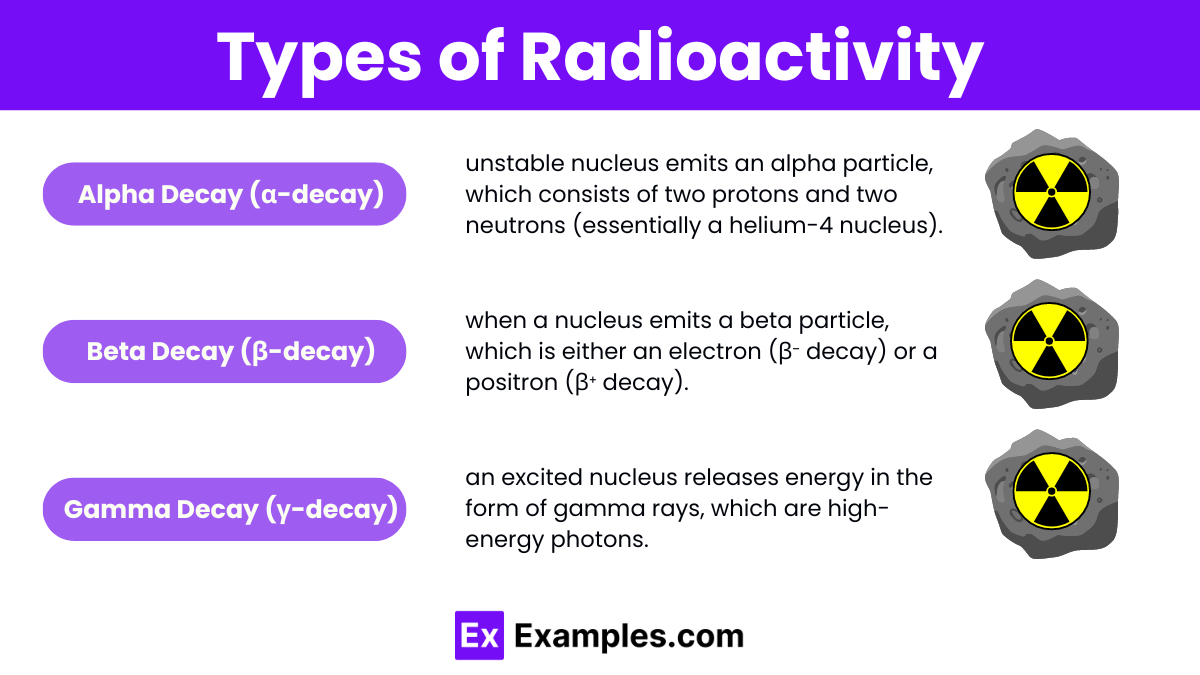
Radioactivity can be classified into four main types based on the nature of the emitted particles or radiation:
Alpha Decay (α-decay):
In alpha decay, an unstable nucleus emits an alpha particle, which consists of two protons and two neutrons (essentially a helium-4 nucleus).
Characteristics: Alpha particles have a relatively large mass and charge, leading to low penetration ability. They can be stopped by a sheet of paper or even human skin but can cause significant damage if ingested or inhaled.
Beta Decay (β-decay):
Beta decay occurs when a nucleus emits a beta particle, which is either an electron (β⁻ decay) or a positron (β⁺ decay).
Characteristics: Beta particles have a smaller mass and charge than alpha particles, allowing them to penetrate further, typically up to a few millimeters of tissue. They can be stopped by materials like plastic or a few millimeters of metal.
Gamma Decay (γ-decay):
In gamma decay, an excited nucleus releases energy in the form of gamma rays, which are high-energy photons.
Characteristics: Gamma rays have no mass or charge, enabling them to penetrate deeply into materials, including human tissue. They require dense materials like lead or several centimeters of concrete for effective shielding.
Neutron Emission:
Neutron emission occurs when an unstable nucleus releases one or more neutrons.
Characteristics: Neutrons have no charge, allowing them to penetrate deeply into materials. They are highly penetrating and require thick layers of materials like water, concrete, or paraffin for effective shielding. Neutron radiation is particularly significant in nuclear reactors and during certain types of nuclear reactions.
Tracking Pollution: Radioactive tracers help track the movement and concentration of pollutants in air, water, and soil, aiding in environmental protection and remediation efforts.
Climate Studies: Isotopes like carbon-14 and tritium are used to study atmospheric and oceanic processes, contributing to our understanding of climate change.
Crime Scene Analysis: Radioactive tracers can be used to detect minute traces of substances at crime scenes, aiding forensic investigations.
Authentication: Radioactive dating techniques help verify the age and authenticity of artifacts and documents.
Deep Space Missions: Radioisotope thermoelectric generators (RTGs) provide long-lasting power sources for deep space probes and rovers, such as the Mars Curiosity Rover.
Cosmic Ray Detection: Instruments on spacecraft use radioactivity to detect and study cosmic rays, providing insights into the composition and behavior of space environments.
Water Tracing: Radioactive isotopes help track the movement and sources of groundwater, improving water management and conservation efforts.
Sediment Dating: Isotopes like lead-210 and cesium-137 are used to date sediment layers in lakes and oceans, aiding in the study of environmental changes over time.
Material Analysis: Radioactive techniques such as neutron activation analysis help identify the elemental composition of artifacts and artworks.
Preservation: Radiation can be used to sterilize and preserve historical artifacts, preventing decay and deterioration.
Luminous Paints: Radioactive materials like tritium and radium are used in luminous paints for watches, instrument dials, and emergency exit signs, providing visibility in low-light conditions.
Antistatic Devices: Radioactive sources are used in some antistatic devices to eliminate static electricity in industrial settings.
Radioactivity has a wide range of applications across various fields due to its unique properties. Here are some of the key uses:
Medical Applications:
Cancer Treatment (Radiotherapy): Radioactive isotopes, such as cobalt-60, are used to target and destroy cancerous cells.
Diagnostic Imaging: Techniques like PET (Positron Emission Tomography) and SPECT (Single Photon Emission Computed Tomography) use radioactive tracers to create detailed images of internal organs and detect abnormalities.
Energy Production:
Nuclear Power Plants: Radioactive materials like uranium-235 and plutonium-239 undergo fission reactions to produce heat, which is then used to generate electricity.
Radioisotope Thermoelectric Generators (RTGs): These devices use the heat released by the decay of isotopes like plutonium-238 to generate electricity, commonly used in space missions.
Industrial Applications:
Material Inspection: Radioactive sources are used in non-destructive testing methods such as radiography to inspect welding joints, pipelines, and structural integrity.
Thickness Gauging: In manufacturing, radioactive sources help measure the thickness of materials like paper, plastic, and metal sheets.
Scientific Research:
Radiocarbon Dating: The decay of carbon-14 is used to determine the age of archaeological and geological samples.
Tracer Studies: Radioactive isotopes are used as tracers to study chemical and biological processes, including the movement of nutrients in plants and the pathways of chemical reactions.
Agricultural Applications:
Food Irradiation: Radioactive isotopes are used to sterilize food, killing bacteria and parasites, and extending shelf life.
Pest Control: Sterile insect technique (SIT) involves releasing irradiated, sterile insects to control pest populations.
Security and Safety:
Smoke Detectors: Americium-241, a radioactive isotope, is used in smoke detectors to ionize air and detect smoke particles.
Border Security: Radioactive sources help scan cargo for hidden contraband and ensure safety at border crossings.
It occurs when atomic nuclei are unstable and undergo decay to achieve a more stable state.
Alpha, beta, and gamma radiation are common types emitted during radioactive decay.
Exposure can damage cells and DNA, potentially leading to cancer or other health issues.
Rocks, soil, and cosmic rays contribute to natural background radiation.
Geiger counters and dosimeters measure radiation levels in counts per minute or sieverts.
It’s the time for half of a radioactive substance to decay into a stable form.
Splitting of atomic nuclei, releasing energy (e.g., in nuclear reactors and bombs).
Radiation is energy emitted from a source, while radioactivity involves the emission of particles or waves from atomic nuclei.
It can affect ecosystems and organisms, depending on exposure levels.
Yes, through processes like neutron bombardment or particle accelerators to create radioactive isotopes.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What type of radiation is most penetrating and requires heavy shielding to stop?
Alpha radiation
Beta radiation
Gamma radiation
Neutron radiation
Which type of radioactive decay results in the emission of an electron from the nucleus?
Alpha decay
Beta-minus decay
Beta-plus decay
Gamma decay
What happens to the atomic number of an element during alpha decay?
It increases by 1
It decreases by 1
It increases by 2
It decreases by 2
Which radioactive decay process does not change the mass number of the element?
Alpha decay
Beta-minus decay
Beta-plus decay
Gamma decay
What is the typical range of alpha particles in air?
A few millimeters
A few centimeters
A few meters
A few kilometers
Which type of radioactive decay involves the emission of a positron?
Alpha decay
Beta-minus decay
Beta-plus decay
Gamma decay
In radioactive decay, what does the term "half-life" refer to?
The time taken for the radioactive element to disappear completely
The time taken for half of the radioactive nuclei to decay
The time taken for the radiation to become harmless
The time taken for the radiation to double in intensity
Which of the following particles is not involved in radioactive decay?
Neutron
Proton
Electron
Photon
Which type of radioactive radiation can be stopped by a sheet of paper?
Alpha radiation
Beta radiation
Gamma radiation
Neutron radiation
What does a Geiger counter measure?
Magnetic fields
Temperature
Radioactive radiation
Electrical current
Before you leave, take our quick quiz to enhance your learning!

