What is the main difference between a solid and a liquid?
Solids flow while liquids don't
Solids have a definite shape, while liquids do not
Liquids have a definite shape, while solids do not
Solids and liquids both have no shape


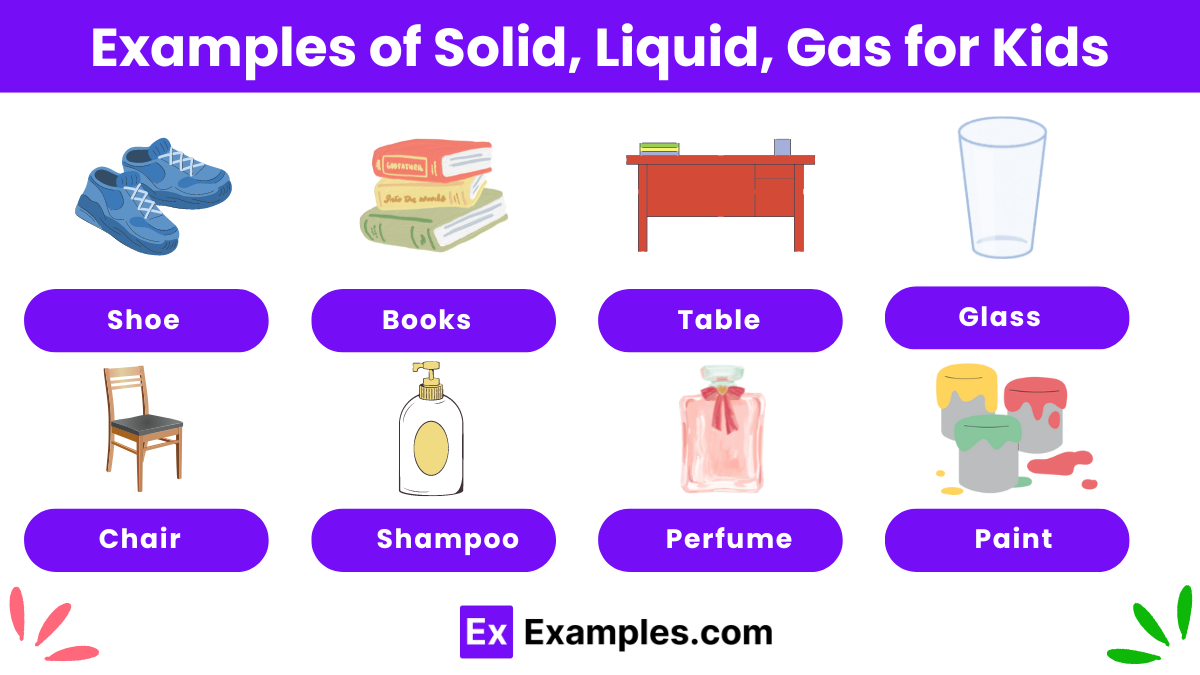
Solids, liquids, and gases are the three main states of matter. Solids have a fixed shape and volume, like a rock or a toy. Liquids have a definite volume but take the shape of their container, like water in a glass. Gases have no fixed shape or volume and can spread out to fill any space, like the air we breathe. Understanding these states helps us see how different materials behave and change in our everyday lives.
Solid, liquid, and gas are the three basic forms of matter. A solid has a fixed shape and volume, like a block of wood. A liquid has a definite volume but takes the shape of its container, like milk in a cup. A gas has no fixed shape or volume and spreads out to fill any space, like the air in a balloon.
Changing between a solid, a liquid, and a gas involves the processes of melting, freezing, boiling, condensation, sublimation, and deposition. These changes occur due to the addition or removal of heat energy, causing changes in the movement and arrangement of particles.
Solids, liquids, and gases differ in their properties:
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Fixed | Takes the shape of its container | No fixed shape, fills any container |
| Volume | Fixed | Fixed | No fixed volume, expands to fill space |
| Particle Arrangement | Closely packed in a regular pattern | Close together but not in a fixed pattern | Far apart and move freely |
| Movement of Particles | Vibrate in place, little movement | Move around each other, allowing flow | Move quickly and freely in all directions |
| Examples | Rock, ice, wood | Water, milk, oil | Air, helium, steam |
Understanding the properties of solids, liquids, and gases helps us comprehend how different materials behave. Here are the key properties of each state of matter:
Solids have a fixed shape, liquids have a fixed volume but no shape, and gases have neither fixed shape nor volume.
Yes, solids can melt into liquids when they are heated, like ice melting into water.
Yes, liquids can evaporate into gases when heated, like water boiling into steam.
Yes, gases can condense into liquids when cooled, like steam condensing into water droplets.
When you freeze a liquid, it turns into a solid because its particles slow down and form a fixed structure, like water freezing into ice.
Yes, solids can sublime into gases without becoming liquid first, like dry ice turning into carbon dioxide gas.
Liquids take the shape of their container because their particles can move around each other, allowing them to flow.
Gases fill their container because their particles move very fast and spread out in all directions.
Solids do not flow because their particles are tightly packed and can only vibrate in place, not move freely.
An example of a liquid is water. It flows and takes the shape of its container.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the main difference between a solid and a liquid?
Solids flow while liquids don't
Solids have a definite shape, while liquids do not
Liquids have a definite shape, while solids do not
Solids and liquids both have no shape
Which state of matter can change its shape but not its volume?
Solid
Liquid
Gas
Plasma
What happens to the molecules in a solid when it is heated?
They move faster and stay close together
They move slower and stay close together
They move faster and spread out
They stop moving
What is the state of matter where molecules are spread out and can fill any container?
Solid
Liquid
Gas
Plasma
Which state of matter has a fixed volume but no fixed shape?
Solid
Liquid
Gas
Plasma
What happens to the molecules in a gas when it is cooled down?
They move faster and spread out
They move slower and come closer together
They move faster and stay the same distance apart
They stop moving
Which state of matter has molecules that are tightly packed and only vibrate in place?
Solid
Liquid
Gas
Plasma
What do you call the process of a liquid turning into a gas?
Freezing
Condensation
Evaporation
Melting
Which state of matter would you expect to find in a balloon filled with air?
Solid
Liquid
Gas
Plasma
What do you call the process of a solid turning directly into a gas?
Condensation
Sublimation
Evaporation
Melting
Before you leave, take our quick quiz to enhance your learning!

