Which property of a material determines its ability to conduct heat?
Specific heat capacity
Thermal conductivity
Thermal expansion
Heat capacity

Thermal properties of matter refer to how materials respond to changes in temperature. Key properties include thermal conductivity (ability to conduct thermal energy), specific heat capacity (heat needed to change temperature), thermal expansion (size change with temperature), and thermal diffusivity (rate of conducting thermal energy). These properties are crucial in fields like engineering, laws of solid state physics, and thermodynamics, influencing how solids behave in different thermal environments.
Thermal properties of matter are characteristics that describe how materials respond to temperature changes. Key properties include thermal conductivity (heat conduction ability), specific heat capacity (heat required for temperature change), thermal expansion (dimension changes with temperature), and thermal emissivity (ability to emit thermal radiation).
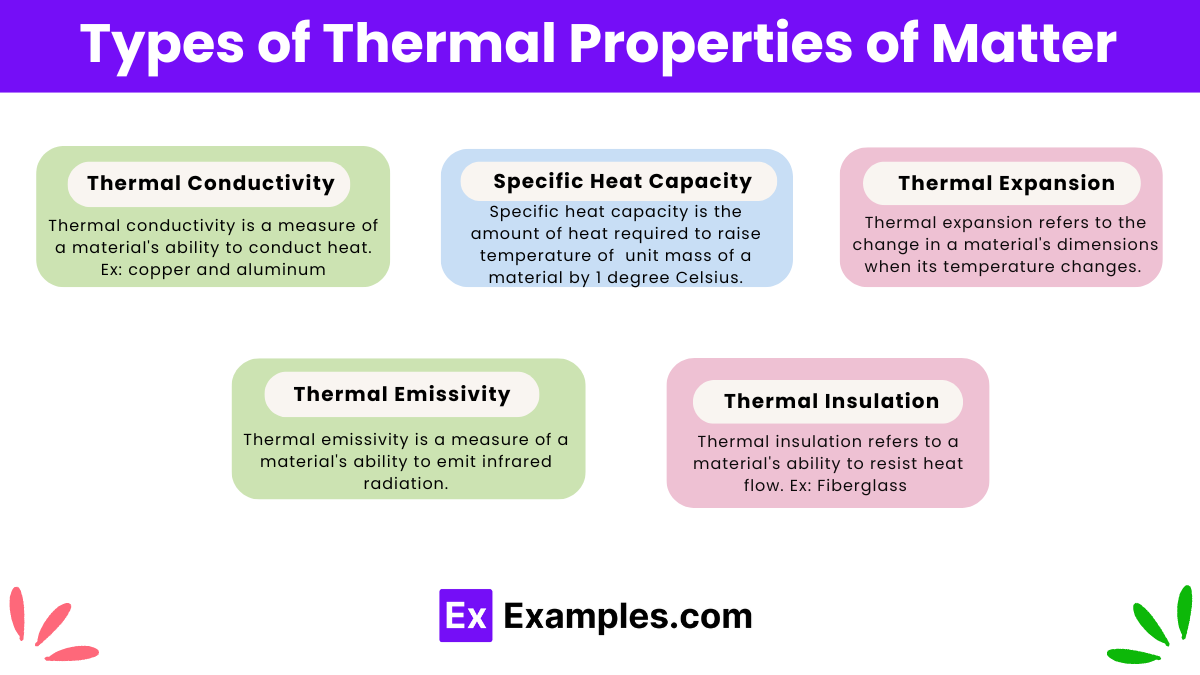
Thermal conductivity is a measure of a material’s ability to conduct heat. Materials with high thermal conductivity transfer heat quickly, while those with low thermal conductivity transfer heat slowly. Examples: Metals like copper and aluminum have high thermal conductivity, making them ideal for cookware and heat exchangers. Insulating materials like rubber and styrofoam have low thermal conductivity, making them useful for thermal insulation.
Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a material by one degree Celsius. Materials with high specific heat capacity can absorb a lot of heat without a significant change in temperature. Examples: Water has a high specific heat capacity, which is why it is used as a coolant in engines. Metals generally have lower specific heat capacities compared to water.
Thermal expansion refers to the change in a material’s dimensions when its temperature changes. Most materials expand when heated and contract when cooled. Examples: Steel railway tracks expand in the summer heat and contract in the winter cold. This is why gaps are left between sections of the tracks to accommodate thermal expansion. Mercury in thermometers expands and contracts uniformly, making it suitable for measuring temperature.
Thermal emissivity is a measure of a material’s ability to emit infrared radiation. It is a surface property and varies between materials. Examples: Black surfaces have high emissivity and are effective at emitting heat, which is why they are used in solar panels. Polished metals have low emissivity and are poor emitters of heat.
Thermal insulation refers to a material’s ability to resist heat flow. Good thermal insulators have low thermal conductivity. Examples: Fiberglass and foam are common thermal insulators used in buildings to reduce heat loss.
Heat capacity is the total amount of heat required to change the temperature of a material. It is an extensive property, meaning it depends on the amount of material. Examples: A large body of water has a high heat capacity, which helps in moderating climate by absorbing and releasing heat.
Thermal stress occurs when a material is subjected to temperature changes, causing expansion or contraction that leads to internal stresses. If the thermal expansion is restrained, it can cause the material to deform or crack. Examples: Glass cookware can crack if exposed to sudden temperature changes due to thermal stress. Metal components in engines must withstand thermal stress caused by rapid heating and cooling cycles.
Solids expand less than liquids and gases because their particles are closely packed and have limited freedom to move compared to particles in liquids and gases.
Heat is the total energy of molecular motion in a substance, while temperature measures the average energy of molecular motion.
Conduction transfers heat through direct contact between particles, where faster-moving particles transfer energy to slower-moving neighboring particles.
Metals like copper, aluminum, and silver are good thermal conductors because they have free electrons that transfer heat efficiently.
Thermal conductivity is a material’s ability to conduct heat. It measures how quickly heat passes through a material.
Convection transfers heat by the movement of fluids (liquids or gases) where warmer, less dense fluid rises, and cooler, denser fluid sinks.
Thermal radiation is the transfer of heat through electromagnetic waves, such as infrared waves, without the need for a medium.
Materials feel colder because they have higher thermal conductivity, drawing heat away from your skin faster.
A thermal insulator is a material that slows down the transfer of heat, like rubber, glass wool, or styrofoam.
Water’s high specific heat capacity helps regulate Earth’s climate by absorbing and storing large amounts of heat.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which property of a material determines its ability to conduct heat?
Specific heat capacity
Thermal conductivity
Thermal expansion
Heat capacity
What is specific heat capacity?
The amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius
The total heat contained in a substance
The measure of a material's resistance to thermal expansion
The energy required to change a substance from solid to liquid
What happens to the volume of most substances when they are heated?
It decreases
It remains constant
It increases
It becomes zero
Which material typically has a high specific heat capacity?
Copper
Water
Aluminum
Silver
What is thermal expansion?
The increase in energy of molecules as temperature increases
The decrease in volume as temperature increases
The increase in volume of a material as temperature increases
The change of state from solid to liquid
Why are bimetallic strips used in thermostats?
To measure specific heat capacity
To conduct electricity
To use thermal expansion to break circuits
To increase thermal conductivity
Which of the following describes latent heat?
Heat absorbed or released during a phase change without changing temperature
Heat required to raise the temperature of 1 gram of water by 1 degree Celsius
Energy stored in the form of potential energy
Energy required to heat a solid substance
What is the boiling point of water at standard atmospheric pressure?
0°C
25°C
100°C
50°C
Which term describes the energy needed to change a substance from solid to liquid?
Specific heat
Latent heat of vaporization
Latent heat of fusion
Thermal expansion
What is the relationship between temperature and thermal energy?
Directly proportional
Inversely proportional
No relationship
Logarithmic relationship
Before you leave, take our quick quiz to enhance your learning!

