What is the SI unit of radioactivity?
Curie
Becquerel
Gray
Sievert

The formula for radioactivity, often referred to as the decay law, quantifies the rate at which an unstable atomic nucleus decays. It is expressed by the equation:
N(t) is the number of undecayed nuclei at time t,
N₀ is the original number of nuclei,
e is the base of the natural logarithm,
λ (lambda) is the decay constant specific to each radioactive substance,
t is the time elapsed.
The units of radioactivity measure the rate at which radioactive decays occur in a sample of radioactive material. The primary unit used is the becquerel (Bq), defined as one decay per second. This unit is part of the International System of Units (SI) and was named after Henri Becquerel, one of the discoverers of radioactivity.
Another commonly used unit, especially in the medical field and older literature, is the curie (Ci), which is larger than the becquerel. One curie is defined as 3.7×10¹⁰ decays per second, which is approximately the activity of one gram of radium-226, a historically significant radioactive substance.
These units quantify the activity of a radioactive sample without directly measuring the energy released during decay. They provide a way to express the stability of the nuclei and the potential intensity of the radiation emitted.
It measures the activity of a quantity of radioactive material and is defined as one decay or disintegration per second.This unit reflects the rate at which unstable atomic nuclei undergo radioactive decay.
This unit was originally based on the activity of one gram of radium-226 and is still commonly used in medicine and older scientific literature, despite the SI system’s preference for the becquerel.
| Unit | Symbol | Equivalent to |
|---|---|---|
| Becquerel | Bq | 1 Bq = 1 disintegration per second |
| Curie | Ci | 1 Ci = 3.7×10103.7×10¹⁰ Bq |
| Rutherford | Rd | 1 Rd = 1 million Bq |
| Gray | Gy | 1 Gy = 1 joule/kg |
| Sievert | Sv | 1 Sv = 1 joule/kg (adjusted for radiation type) |
| Rad | Rad | 1 Rad = 0.01 Gy |
| Rem | Rem | 1 Rem = 0.01 Sv |
The SI unit of radioactivity, symbolized as Bq. It is defined as one disintegration or decay per second. This unit directly measures the activity of a radioactive substance without reference to the energy of the radiation emitted.
A traditional unit of radioactivity, symbolized as Ci. One curie is equivalent 3.7×10¹⁰ to becquerels. The curie is based on the radioactivity of one gram of radium-226 and is still widely used in medicine and industry.
Less commonly used today, this unit is symbolized as Rd. One Rutherford equals one million becquerels (1 million disintegrations per second), and it was historically used to measure significant levels of radioactive activity.
A unit of absorbed radiation dose, symbolized as Gy. One gray is the absorption of one joule of radiation energy per kilogram of matter. It quantifies the amount of radiation energy absorbed by the tissue.
The unit of radiation dose equivalent, symbolized as Sv. It measures the health effect of ionizing radiation on human tissue, taking into account the type and energy of the radiation. One sievert equals one joule per kilogram, adjusted for biological effectiveness.
An older unit of absorbed dose, where one rad is equivalent to 0.01 gray (100 rad = 1 gray). Symbolized as Rad, it is still used in some contexts within the United States.
Similar to the sievert, the rem (Roentgen equivalent man) measures the biological effects of ionizing radiation. Symbolized as Rem, one rem is equivalent to 0.01 sievert.
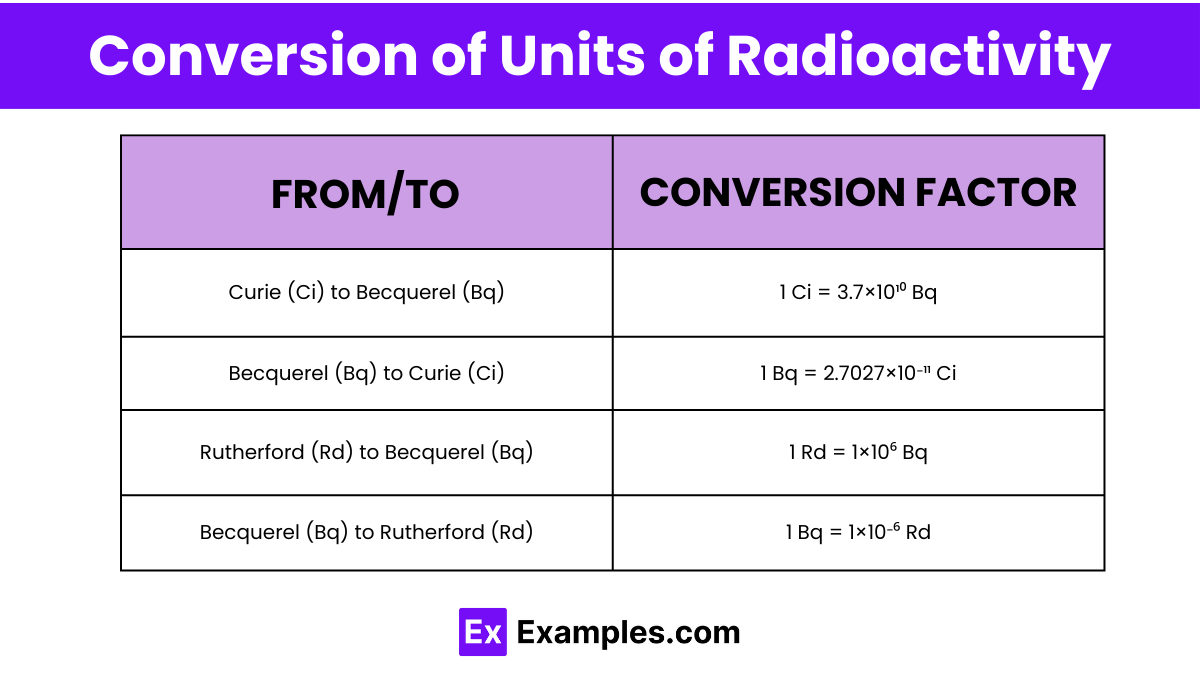
| From Unit | To Unit | Conversion Factor |
|---|---|---|
| Curie (Ci) | Becquerel (Bq) | 1 Ci = 3.7×10¹⁰ Bq |
| Becquerel (Bq) | Curie (Ci) | 1 Bq = 2.7027×10⁻¹¹ Ci |
| Rutherford (Rd) | Becquerel (Bq) | 1 Rd = 1×10⁶ Bq |
| Becquerel (Bq) | Rutherford (Rd) | 1 Bq = 1×10⁻⁶ Rd |
1 curie is approximately equal to 37 billion becquerels. This conversion factor is used to translate between the traditional unit (curie) and the SI unit (becquerel) of radioactivity.
1 becquerel is approximately equal to 2.7027 x 10^-11 curies. This allows for the conversion from the SI unit back to the traditional unit of radioactivity.
1 Rutherford, a less commonly used unit, is approximately equal to 1 million becquerels, used mainly in historical or specific scientific contexts.
This conversion allows for the transition from the SI unit of radioactivity to the older, less common unit of Rutherford, facilitating understanding and calculations in diverse contexts.
The seven types of radiation include alpha, beta, gamma, X-ray, neutron, positron, and proton radiation, each with unique properties and interactions with matter.
Radioactivity is measured using devices like Geiger counters, scintillation counters, and ionization chambers, which detect and quantify radioactive decay particles and radiation.
Multiple units for radiation exist to address different measurement needs, historical developments, and scientific and medical applications, enhancing precision and context relevance.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the SI unit of radioactivity?
Curie
Becquerel
Gray
Sievert
What unit is used to measure the absorbed dose of radiation?
Becquerel
Curie
Gray
Sievert
Which unit is commonly used to measure radiation exposure to human tissue?
Becquerel
Curie
Gray
Sievert
What does the unit \"Curie\" measure?
Absorbed dose of radiation
Radiation exposure to human tissue
Activity of a radioactive substance
Energy of radiation
What is the unit for measuring the energy imparted by ionizing radiation to matter?
Becquerel
Curie
Gray
Sievert
What is the relationship between the Gray and the Sievert?
They measure the same quantity
1 Gy = 1 Sv for all types of radiation
1 Gy = 1 Sv only for gamma rays
1 Gy = 1 Sv for alpha particles
What unit is used to express the activity of a radioactive source in terms of disintegrations per minute?
Becquerel
Curie
Gray
Sievert
Which unit is used to measure the dose equivalent in radiological protection?
Becquerel
Curie
Gray
Sievert
How is 1 Becquerel defined?
One disintegration per minute
One disintegration per second
One joule per kilogram
One Curie
What is the unit of measurement for radiation exposure in the context of environmental radioactivity?
Becquerel
Curie
Gray
Sievert
Before you leave, take our quick quiz to enhance your learning!

