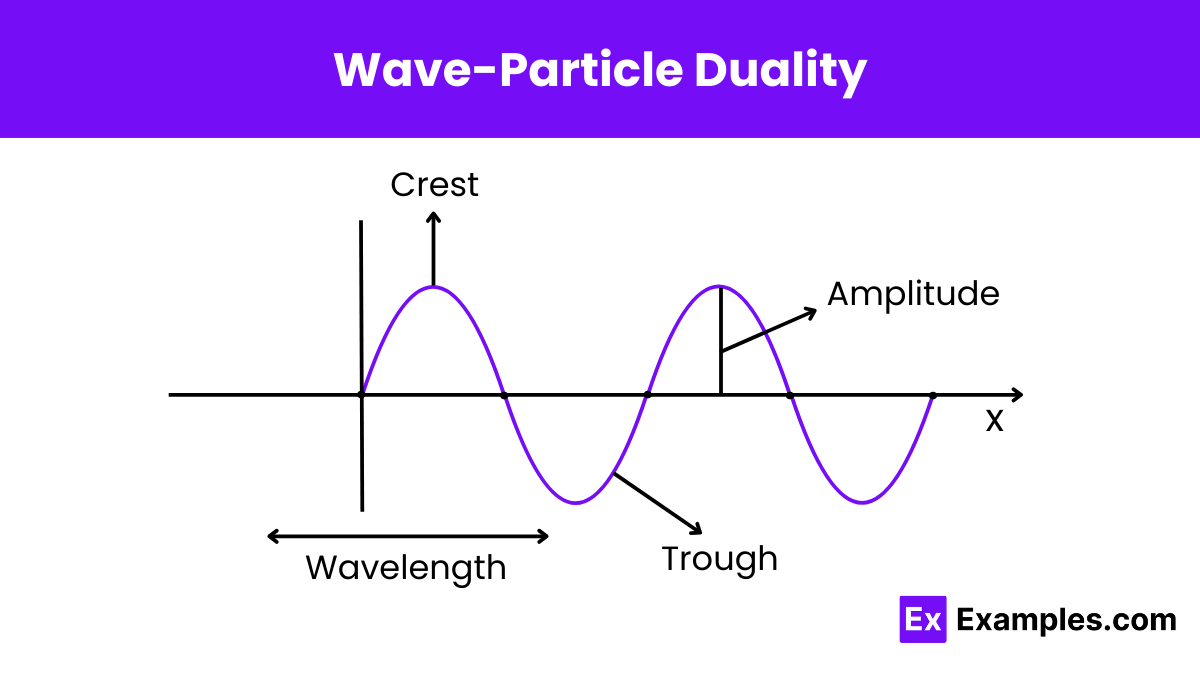
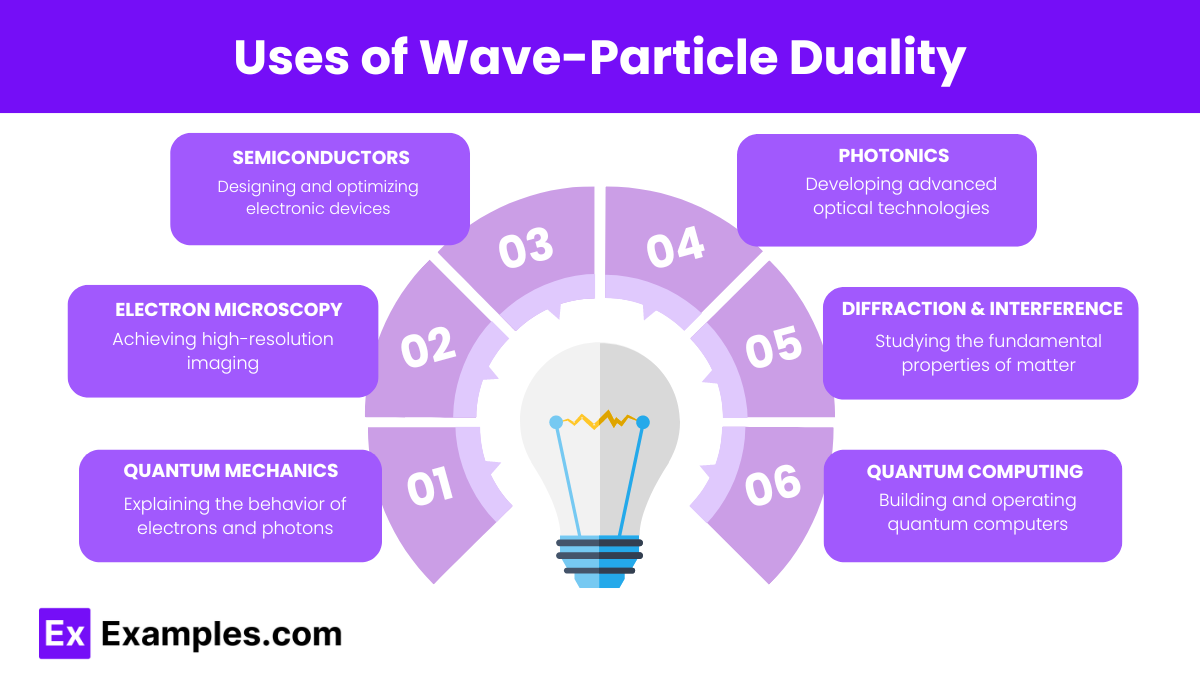
What does wave-particle duality suggest about the nature of light?
Light can be purely described as a wave or as a particle, but not both.
Light exhibits properties of both waves and particles depending on the experiment.
Light is always a particle and never behaves as a wave.
Light behaves as a wave and particle simultaneously without any experimental evidence.